A novel signal amplification strategy of an electrochemical immunosensor for human chorionic gonadotropin, based on nanocomposites of multi-walled carbon nanotubes–ionic liquid and nanoporous Pd
Abstract
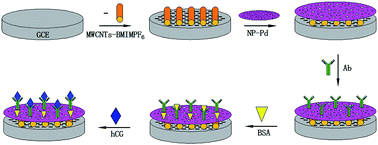
A sensitive label-free immunosensor adopting a novel signal amplification strategy was proposed for the electrochemical detection of human chorionic gonadotropin (hCG). Firstly, a novel composite film consisting of multi-walled carbon nanotubes (MWCNTs) and a room temperature ionic liquid (RTIL) of 1-butyl-3-methylimidazolium hexafluorophosphate (BMIMPF6), which combined the advantages of MWCNTs and RTILs, was fabricated on a glassy carbon electrode surface. The mechanism of the synergy between the MWCNTs and RTIL has been discussed. Secondly, the first film was modified with nanoporous Pd (NP-Pd) prepared by a simple dealloying method. The structure of NP-Pd has been confirmed by EDS, XRD, SEM, TEM and BET analysis. Due to the large specific surface area and excellent electrical conductivity of NP-Pd, electron transfer was promoted and the amount of hCG antibody was enhanced significantly. The results showed that MWCNTs–BMIMPF6/NP-Pd composites were successfully designed as a sensitive immunosensor platform for hCG determination. Under the optimum conditions, the immunosensor exhibited high sensitivity and a wide linear range for hCG from 0.05 to 50 ng mL−1 with a detection limit of 3.2 pg mL−1. The prepared immunosensor showed high sensitivity, reproducibility and stability. This immunosensor preparation strategy presents a promising platform for clinical application.

 Please wait while we load your content...
Please wait while we load your content...