Fluorometric appraisal of HSO4− in aqueous media and daily utilities using organic–inorganic nanohybrids†
Abstract
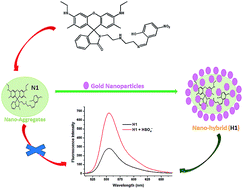
Receptor 1 based on rhodamine dye was designed and characterized using 1H, 13C NMR and mass spectroscopy like spectroscopic techniques. Re-precipitation methods have been used for developing nano-aggregates of 1 (N1) in aqueous medium, which were further used for preparation of inorganic–organic hybrid nanomaterial (H1) i.e. by coating organic nanoparticles with gold nanoparticles. Changes in the photophysical profile by anion binding of N1 and H1 were evaluated using fluorescence spectroscopy. No selectivity was perceived with nano-aggregates (N1) for any anion. However, the hybrid nanomaterial (H1) selectively recognized HSO4− through enhancement of fluorescence emission intensity in aqueous medium. Thus, the hybrid nanomaterial (H1) has been established for sensing of HSO4− and was considered for various parameters like rapid response, good stability in water and is validated for determination of HSO4− ions in daily use items.

 Please wait while we load your content...
Please wait while we load your content...