SnO2@Co3O4 p–n heterostructures fabricated by electrospinning and mechanism analysis enhanced acetone sensing†
Abstract
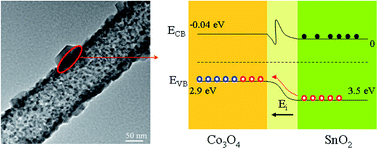
SnO2@Co3O4 p–n heterostructure nanotubes have been obtained for the first time via precursor nanofibres and subsequent annealing. The structure and properties of heterostructure nanotubes were characterized using various analysis methods. The responses of composites with different SnO2 content to 10 ppm acetone have been measured, and found that the composites exhibited higher sensitivity, lower operating temperature and faster response than pure Co3O4. The improvement of sensing characteristics is attributed to the formation of a p–n heterojunction between the two types of semiconductor oxides.

 Please wait while we load your content...
Please wait while we load your content...