An effective fluorescent probe to detect glutathione from other sulfhydryl compounds in aqueous solution and its living cell imaging†
Abstract
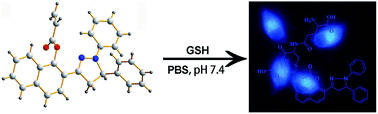
We designed and synthesized a two-photon fluorescent probe, 2-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)naphthalen-1-yl acrylate (PPN) with improved properties based on the naphthalene–pyrazoline fluorophore. The probe exhibits high sensitivity to glutathione (GSH) in PBS–CTAB buffer solution with a low detection limit of 1.5 × 10−8 M and a response time less than 10 min. According to the HRMS and fluorescence spectra analysis, the detection mechanism was confirmed to be a Michael addition reaction induced by the sulfhydryl of GSH. In addition, probe PPN has very good selectivity and is able to discriminate GSH from cysteine (Cys), homocysteine (Hcy) and other sulfhydryl compounds with bright two-photon-excited fluorescence. Moreover, we have successfully applied PPN to calf serum samples and living cell imaging with good effect.

 Please wait while we load your content...
Please wait while we load your content...