Progress in the Suzuki polycondensation of fluorene monomers†
Radu-Dan Rusu*ab and
A. Dieter Schlüterb
a“Petru Poni” Institute of Macromolecular Chemistry, Romanian Academy, Aleea Grigore Ghica Voda 41A, Iasi-700487, Romania. E-mail: radu.rusu@icmpp.ro
bLaboratory of Polymer Chemistry, Institute of Polymers, Department of Materials, ETH Zurich, Vladimir-Prelog-Weg 5, HCI J541, CH-8093 Zurich, Switzerland
First published on 17th October 2014
Abstract
A step-growth AA/BB type Suzuki polycondensation approach has been applied to synthesize two high molecular weight polyfluorenes bearing nitrile-terminated short alkyl side chains and protected amino functionalities. The polymers were obtained reproducibly on the gram scale and as typical polyfluorene-based materials. Three different often used palladium catalysts and several reaction parameters were evaluated in order to optimize the conditions towards high molar masses. The issues encountered during the polymerizations led to a thorough investigation by in-depth mass spectrometric analysis of the oligomeric species obtained as side products. This resulted in some mechanistic insights showing ligand scrambling as the major side reaction. The decorated polyfluorenes reported here seem to be the best choice so far for post-polymerization modifications performed on high molecular weight conjugated polymers with lateral functional groups.
Introduction
The discovery1 of the Suzuki–Miyaura cross-coupling reaction provided one of the missing pieces in the great puzzle of unsolved macromolecular synthetic challenges – an efficient and relatively gentle pathway to directly connect aromatic units to one another.2 Soon after,3 it was transformed into a robust synthetic protocol, the so-called Suzuki polycondensation (SPC), in order to fill up a blank space on the list of wanted polymers – fully conjugated polyarylenes. Two decades later, SPC has reached considerable maturity and evolved into a powerful and widely used polymerization tool for a broad variety of conjugated macromolecules with a wide range of substitution patterns.4Since this polymerization route provides access to well-engineered materials, numerous developments in SPC have been provided in recent years leading to prominent improvements in catalytic efficiency, product yield and ease of manipulation.5 Driven by the inherent beauty of their unique molecular structures and their commercially relevant properties,6 extensive attention has been paid to polyfluorenes, by far most of the optimization being performed in industry. However, with some notable exceptions,7 most reported SPC protocols still use only small modifications of the original recipe, often conducted under poorly optimized conditions. Consequently, the full potential of the method has not been exploited and products are often oligomeric or of low molecular weight (in the range of 1 to 10 kDa). One reason for this deficiency is obvious: as far as the technologically relevant optical properties are concerned, polymer-typical properties can already be obtained for relatively short chains. Nevertheless, material features can be extremely sensitive to the length of the polymer chains, and a polymer-based device will finally fail if it softens, fuses or cracks under operating conditions.
In the present study, we explored the compatibility range of SPC to polyfluorenes with high molecular weights by using the widely employed and lucrative AA/BB approach which involves two different monomers carrying either boronic or bromide functionalities.
A literature survey afforded only few results on fluorene monomers bearing nitrile8,9 or (protected) amino10–12 functionalities submitted to SPC. A very good review on this topic actually asserted that “Suzuki polycondensation cannot be used” for the former,13 a statement presumably true at the time it was written. At a first glance, this assertion still stands, for both systems even, since we only came across a few publications reporting merely low molar mass (co)polymers, or, if considering the average degrees of polymerization and the significant molar mass overestimation by GPC studies with polystyrene calibration,3 higher oligomers.
Therefore, this article deals with this oversight by reporting medium to large molar mass polyfluorenes based on monomers with the above mentioned lateral functional groups. In order to achieve them, an investigation of the effect of several reaction parameters on resulting molecular weights was performed and some mechanistic insights gained by in-depth mass spectrometric analysis.
Results and discussion
The AA/BB type approach is generally considered to be a step-growth process which usually involves the combination of two aromatic, symmetric monomers and leads to macromolecules containing the two aromatic segments in an alternating fashion. In the present work, this SPC coupling approach has been applied to synthesize two relatively similar fluorene polymers bearing nitrile-terminated short alkyl side chains (the CN polymer) and protected amino functionalities (the NHBoc polymer), respectively (Scheme 1).These particular monomers were selected in order to mediate an envisaged good solubility of the growing species during SPC and of the subsequent final products and also for the feasibility of future post-polymerization modifications, like quantitative deprotection and dendron attachment.14 In addition to the Boc protecting group, nitrile moieties can be considered excellent masking groups for amino moieties during SPC. As second reaction partner, we chose an already classic diboronic derivative, the 1,4-benzenediboronic acid bis(pinacol).
The synthesis of the monomers was performed by following known procedures11,15,16 which after up-scaling and optimization provided them in relatively large batches (20 g for the two bromides, 50 g for the boronic ester) and overall yields above 75% (ESI†). The required as high as possible degree of purity for these monomers was achieved by repeated recrystallizations and was quantified by high resolution NMR spectroscopy – purity being a key issue for SPC, especially as far as the AA/BB approach is concerned. The purity was assessed to be ca. 99.5% by using the 13C NMR satellite peaks as internal reference.17
SPC conditions towards high molecular weights
The protected-diamine-decorated fluorene monomer was first submitted to SPC with the bis-pinacolato derivative (Scheme 1) under conditions previously reported as yielding high molecular weights for the AA/BB approach: Pd[P(p-tolyl)3]3, NaHCO3 in THF–water, 70 °C for 96 hours under nitrogen.18 The gel permeation chromatographic (GPC) analysis of the obtained products generated a monomodal broad elution curve unexpectedly positioned around 25 kDa. We considered this a troublesome low molecular weight regime by recalling the earlier mentioned average degree of polymerization and the overestimated values usually generated for rigid-rod polymers by polystyrene calibration. This disappointing formation of higher oligomers was ascribed to the poor solubility of the growing macromolecular species which resulted in early precipitation, a behavior that was also observed when CH2Cl2 was used as a solvent.The situation changed when we switched to the higher boiling point solvent toluene which allowed for a higher reaction temperature (90 °C). This resulted in higher molar mass products: Mw of 110 kDa and Mn of 58 kDa. Encouraged by this finding, several trial polycondensations were performed in order to find the best-suited catalyst for this system, by testing two other widely used, commercial Pd-based catalyst precursors: the classic tetrakis(triphenylphosphine)-containing catalyst Pd0(PPh3)4 (self-prepared and used freshly so as to ensure high purity), and the bulky, more stable ferrocene-containing catalyst PdII(dppf)Cl2 (used as received, due to its high air stability). We screened these three widely used catalytic systems together with other parameters involved in this protocol so as to achieve the maximum catalytic efficiency and to determine the best compromise between several reaction conditions (solvent, temperature, monomer concentrations, catalyst loading) in order to promote highly quantitative monomer conversion. During all experiments two parameters were kept stable: the reaction time, which was 96 h for each run, and the base – the not so powerful NaHCO3. In order for these results to actually gain relevance,4b the experiments were made starting from a minimum 500 mg amount of the fluorene monomer and the results represent the average of at least two runs.
The best results that were obtained so far in the case of the amino-based system (Table 1) are encouraging: Mw = 262 kDa and Mn = 110 kDa. They were obtained by employing the following conditions: 1 mol% Pd(dppf)Cl2, 8.5% monomer concentration, NaHCO3 in toluene–water = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 90 °C for 96 hours under nitrogen.
2, 90 °C for 96 hours under nitrogen.
| Polymer | Catalyst | Mw (kDa) | Mn (kDa) | Pn | PDI | Yield% |
|---|---|---|---|---|---|---|
a Polymerizations were set up with 1 mol% catalyst concentration, toluene–water = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, NaHCO3, 90 °C for 96 hours under nitrogen, 8.5% monomer concentration for NHBoc and 5% for CN. Reported values were obtained starting from a minimum 500 mg amount of fluorene monomer and represent the averages of at least two trials. 2, NaHCO3, 90 °C for 96 hours under nitrogen, 8.5% monomer concentration for NHBoc and 5% for CN. Reported values were obtained starting from a minimum 500 mg amount of fluorene monomer and represent the averages of at least two trials. |
||||||
| NHBoc | Pd[P(p-tolyl)3]3 | 110 | 58 | 104 | 1.9 | 89 |
| Pd(dppf)Cl2 | 262 | 110 | 198 | 2.4 | 78 | |
| Pd(PPh3)4 | 150 | 80 | 144 | 1.9 | 86 | |
| CN | Pd[P(p-tolyl)3]3 | 108 | 36 | 96 | 3.0 | 72 |
| Pd(dppf)Cl2 | 60 | 29 | 77 | 2.1 | 83 | |
| Pd(PPh3)4 | 62 | 26 | 69 | 2.4 | 82 | |
An impressive example of the catalytic efficiency of the examined systems is given in Fig. 1(left). It can be noted that the chromatographic profile – a relatively broad monomodal elution curve – is quite similar for all three catalytic systems, with a larger polydispersity index (PDI = 2.4) for the highest molar mass obtained with Pd(dppf)Cl2 as compared to the other two (both PDIs = 1.9).
 | ||
| Fig. 1 GPC elution curves (normalized to maximum intensity) for polymerizations performed with different catalysts (solvent: DMF + 1% LiBr). | ||
The highest molecular weights obtained for the NHBoc polymer were all generated by SPC experiments that gelled during polymerization. As noted previously,5a this behavior could be a Trommsdorff-like effect in which the potential, molar mass limiting side-reactions are more affected by the increased viscosity than the desired high conversion polymerization process.
Almost all of the above observations proved inapplicable to the case of the CN polyfluorene, for which toluene was still the solvent of choice. The catalytic efficiency completely changed, with the highly reactive, coordinatively unsaturated Pd[P(p-tolyl)3]3 providing the highest turnover catalysis (Table 1), while the classic tetrakis(triphenylphosphine)- and the bulky, ferrocene-containing catalysts gave acceptable, yet clearly inferior results to the former. An eloquent example of the catalytic efficiency for the CN polymer is displayed in Fig. 1(right).
While exploring the SPC potential of this system, we came across an impediment that led to large deviations from expected values – regardless of the catalytic system used. Bimodal GPC elution curves were obtained in each case, with the lower molecular weight fraction being situated more or less at the same retention time and displaying the highest peak intensity. When evaluating the effect of the catalytic systems, it can be observed that the catalyst with highest turnover, Pd[P(p-tolyl)3]3, generated the broadest PDI of the high molecular weight fraction but also the oligomeric fragment with the highest molar mass and lowest peak intensity when compared to the other two.
After further screening regarding catalyst and monomer concentration, we obtained a satisfactory high molar mass of Mw = 108 kDa and Mn = 36 kDa, by applying the following parameters: 1.5 mol% Pd[P(p-tolyl)3]3, 5% monomer concentration, NaHCO3 in toluene–water = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 90 °C for 96 hours under nitrogen. It must be mentioned that these values were obtained for the high molar mass part of the bimodal GPC curve.
2, 90 °C for 96 hours under nitrogen. It must be mentioned that these values were obtained for the high molar mass part of the bimodal GPC curve.
While the highest molecular weight NHBoc polymers gelled during polymerization, the corresponding CN polymers precipitated during the last hours of the reaction, a factor which could also be a limitation for achieving higher molar masses.
Therefore, it is reasonable to assume that the differences between the molecular weights of the two evaluated polymeric systems, CN and NHBoc, come from their different solubilities, with the amine-decorated polymer having an advantage in this regard due to the chemical nature of the protecting group. In addition, the molar mass limiting effect of the side reactions occurring in the case of the CN polyfluorene should be also taken into consideration. Further use of previously effective15 phase transfer catalysts like tetraalkylammonium salts, in order to promote higher molecular weights for both polymeric products or to quantitatively reduce the high oligomeric fraction for the second one, did not lead to a visible improvement.
In order to actually understand the relevance of the reported results, they need to be compared to the ones reported in the literature for some similar systems: Mw = 56 kDa for the amine-decorated polymer,11 and Mw = 28 kDa for the second, nitrile-related one.9 The latter result corresponds to a system with an even more extended repeat unit due to a longer alkyl chain, which makes the discrepancy between the two even bigger. This crude comparison shows the synthesized polyfluorenes to have the highest molecular weights among those with this type of substitution patterns and (strikingly) superior values as compared to usually reported polyfluorenes.
If higher molecular weight polyfluorenes are needed, which are scarcely reported in the literature4,6 and are mostly generated by industrial optimization of octyl- or 2-ethylhexyl-bearing fluorenes of higher solubility, the polymers reported here may not be the ones of choice. An outstanding molecular weight of 1.4 MDa obtained from “extraordinary pure” spirofluorene monomers7c should be mentioned in this regard. However, if one looks for post-polymerization chemistry performed on high molecular weight polyfluorenes, the decorated systems reported here seem to be the best choice so far, thus increasing the intrinsic potential of SPC.
At this point, a comment regarding polymerization yields is appropriate. The discussed respectable molar masses refer to polymers which were isolated in yields of well below 100% (72% for the highest molar mass CN polymer and 78% for the NHBoc polymer). They therefore do not seem to follow the Carothers equation according to which a high molar mass polymer can only be reached for extremely high conversions, which normally should go hand in hand with high yields. There are two reasons for the low yield values. First, an insoluble material was obtained in all polymerizations, which was filtered off and collected separately, accounting for ca. 10% weight for the highest molar mass CN polymer and 8% for the NHBoc polymer. The amount of this insoluble material, whose nature or molecular weight we were not able to determine without any doubt, decreased with lower molecular weight polymers. Second, all high-turnover polymerizations were accompanied by strong foaming which caused higher than common material losses despite a very careful workup and several extractions of the inorganic phases. Moreover, some small losses could come from inadvertent fractionation due to low molar mass linear or cyclic products, which do not precipitate during polymer recovery.
While we believe to have reached a quite satisfactory state, there is still room for further research and engineering regarding the challenge of achieving even higher molecular weights and yields for the envisaged polymeric structures. Further optimization considering the effect of more exotic catalytic systems or different bases5a,b upon the degree of monomer conversion could be considered. A very recent article7d reports similar molecular weights obtained by means of chain-growth AB-type SPC applied on classic 2-ethylhexyl-decorated fluorene monomers. Moreover, it proves AB-type SPC to work faster, with much less catalyst and with superior (8 times higher) molar masses as compared to the AA/BB one. It would be interesting to see if these conclusions also apply for the polymers described herein, and the first steps in this direction are already in progress.
Structural analysis of the oligomeric SPC products. Mechanistic insights
As mentioned, during the polymerization of the nitrile-decorated fluorene monomer, a bimodal GPC curve was always obtained, despite changing the catalyst system, a behavior which is intuitively expected to be determined by side reactions characteristic to this synthetic protocol. If this issue can be considered negligible in low molecular weight Suzuki–Miyaura cross-coupling – the side-products being easily removed – in polymer synthesis side reactions cannot be accepted since they could be responsible for undesired defects in the polymeric product. We therefore performed an in-depth analysis of the high oligomers by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) in order to elucidate the nature of the occurring side reactions.A lower mass CN product, chosen due to its improved solubility as compared to the higher analogues, was subjected to preparative GPC in order to separate it into two fractions at the saddle point between the high and low molecular weight peaks from the GPC chromatogram (Fig. 2).
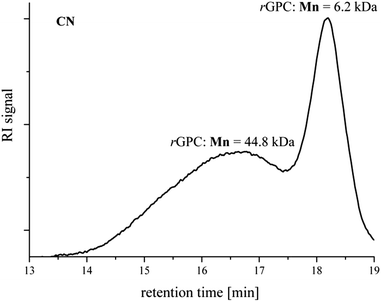 | ||
| Fig. 2 GPC elution curve of the CN SPC product as subjected to preparative GPC. The Mn values represent the number-average molecular weights of the separated products. | ||
This technique gave us a high fraction with a molecular weight of Mn = 45 kDa and an oligomeric one with Mn ∼ 6 kDa. The latter was resubjected to preparative GPC in order to further separate the individual components as far as possible, an initiative which proved unsuccessful. Since the assignment of the end group protons from the 1H NMR spectra of this low fraction was very difficult, if not impossible, the sample was analyzed by MALDI-TOF MS.
Based on previous work, there are two main possibilities that could generate such low molar mass products: either cyclic oligomers or linear, open-ended species. Both of them arise from undesired side reactions like cyclization, hydration, hydroxylation, homocoupling, ligand scrambling and deboronation which can in principle take place at both boron and bromo termini.16,17,19
The mass spectrum gives a molar mass around 2.5 kDa, proving once more the overestimation of GPC performed using polystyrene standards. It exhibits several series of signals (Fig. 3), which were determined to belong to different linear, open-ended oligomeric species. No signals of cyclic products were found.
Further analysis revealed 10 distinct clusters of homologous peaks separated by 374.0–374.3 Da, which correspond to the mass of the polyfluorene repeat unit (374.18 Da; designated as M in Table 2).
| Pattern | End groups | Number of repeat units |
|---|---|---|
| A | (HO)2B–Mn–P(Tol)2 | n = 2–5 |
| B | (Tol)2OP–Mn–PO(Tol)2 | n = 2–4 |
| C | Tol–Mn–PO(Tol)2 | n = 3–5 |
| D | H–Mn–Fl–Br | n = 3–5 |
| E | (Tol)2OP–Mn–FlBr | n = 3–4 |
| F | Br–Mn–Tol | n = 3–5 |
| G | (HO)2B–Ph–Mn–Br | n = 3–4 |
| H | TolP–Mn–PO(Tol)2 | n = 3 |
| I | H–Mn–Tol | n = 3–4 |
| J | (Tol)2OP–Mn–B(OH)2 | n = 2–5 |
Each cluster was assigned to linear chains containing between 2 and 6 repeat units bearing different end groups. Calibrated mass spectra allowed a pertinent assignment of almost all families of peaks, and the corresponding complex structures are schematically displayed in Table 2. A more straightforward, intuitive interpretation of this analysis is presented in Fig. S4 in ESI† by comparing the MALDI-TOF mass spectrum of the lower mass fraction with a simulated one for all the discussed patterns.
Besides the judiciously identified molar masses, the presence of the families of peaks belonging to oligofluorene chains bearing hydrolyzed boronic esters (patterns A, G and J) or bromide-terminated monomer fragments (D, E, F and G) as at least one of the end groups confirms the linearity of these chains.
The mass spectrum shows other families of fluorene oligomers terminated with unreactive units at both termini, such as Tolyl2/Tolyl2 (pattern B in Table 2), Tolyl/Tolyl2 (species C and H) and H/Tolyl (cluster I). These H- and Tolyl-terminated chains clearly disclose the occurrence of simultaneous side reactions like debromination, hydrolytic deboronation and, most of all, ligand scrambling during the SPC protocol, in accordance with previously reported data.20 As can be easily observed, most of these open-ended species (except for D and G patterns) are decorated with at least one end group coming from the ligand, which originate from ligand scrambling.21 In our case, the occurrence of these reactions does not seem to be influenced by the nature of the employed ligand or Pd source, since all macromolecular compounds display bimodal GPC elution curves, regardless of the catalytic system used. Ligand scrambling is an aryl–aryl exchange between the Pd center and phosphorus from the ligand. As a consequence, arylboronic acids will couple not only with the provided aryl halide but also with aryls of the phosphine ligand. Since almost all end groups of the detected products come from ligand scrambling, it is concluded that this is the major side reaction which requires attention if further optimization is intended.
There is no clearly dominant peak series but the most intense one belongs to a symmetrically ended oligomer containing PO(Tolyl)2 termini.
It is reasonable to assume that the side reactions are taking place right at the beginning of the reaction, and involve only short chains, and, once a certain chain length is achieved, like more than 7 or 8 repeat units, their influence upon the entire process is diminished and the reaction proceeds in a normal, expected step-growth fashion. While the comprehensive investigation of these oligomeric species resulted in a valuable insight into the mechanism, all performed optimization attempts did not bring any change to this early halt in growth due to side reactions.
Properties of the highest molar mass polyfluorenes
The CN and NHBoc polymers with the highest molecular weights were further investigated in regard to solubility, film-forming ability, thermal stability and optical properties.The solubility was qualitatively tested in different solvents, satisfactory results being obtained for high-boiling solvents like dimethylformamide, 1-methyl-2-pyrrolidone, tetrachloroethane and toluene at elevated temperatures. While this need for thermal treatment is a disadvantage compared to similar NHBoc products with much lower molecular weights,11 in turn it proves the much higher molar masses.
Efforts towards formation of fibers and films have not been satisfactory yet because of the limited solubility of the polymers under ambient conditions. Investigations at elevated temperatures are in progress.
The thermal stability of these polymers was investigated by thermogravimetric analysis under nitrogen atmosphere. First of all, it was observed that even after drying under high vacuum for 48 h, the polymers still retain a small percentage of water and toluene, the starting solvent mixture for the reaction, as can be observed from a small weight loss around 100 °C. Similar phenomena were previously observed.12,22
Further it was found that these polymers are thermally stable up to 200 °C, in the case of the amino system, and 250 °C for the nitrile one. Above these temperatures, different thermal decomposition processes occur, every one of them being identified by further investigations.
After solvent evaporation, the nitrile-decorated CN system showed two distinct thermal decomposition steps (Fig. 4), which correspond to the cleavage of the nitrile group and the rupture of the short alkyl chains, two processes that seem to be overlapping in this region.
In order to confirm this, the sample was heated at 300 °C for 24 h, and the infrared spectra before and after the thermal treatment were measured (Fig. 5). After this treatment the nitrile characteristic band disappeared almost completely while some of the methylene corresponding bands were severely diminished, thus confirming that the two processes are overlapped.
 | ||
| Fig. 5 IR spectra of polymer CN before and after thermal treatment at 300 °C for 24 h (the * marked peaks correspond to solvent characteristic peaks). | ||
The second system provided easier-to-resolve TGA curves (Fig. 6), with two distinct decomposition stages. They correspond to the thermal degradation of the Boc protecting groups and to the cleavage of the amino-terminated alkyl chains.
The optical properties of the CN and NHBoc fluorene-based polymers were investigated by absorption and emission spectroscopy in DMF solutions. The absorption spectra (Fig. 7) consist of a strong, featureless π–π transition which peaks at 370 and 380 nm respectively, especially characteristic for polyfluorenes but also for many other π-conjugated polymers.23
 | ||
| Fig. 7 Absorption (top) and fluorescence (bottom) spectra of polymers NHBoc and CN in DMF solutions (10−5 M). | ||
The emission spectra of these polymers show typical, vibronic well-resolved structures with peaks at 410, 430 and 470 nm, assigned to different intrachain singlet transitions, with the 0–0 transition being the most intense one. These violet to blue spectra, with a small greenish tail emission, are typical for polyfluorenes and represent one of their key features.23
The fluorescence spectra of these polymers were recorded at different concentrations, from 10−5 to 10−9 M in DMF (Fig. S5 in ESI†). The intensity of this blue emission increased proportionally with the concentration and no serious change in their peak maxima or shape was observed, indicating that no self-absorption takes place.
We also measured the fluorescence quantum yields relative to the universal diphenylanthracene standard or to the more specific fluorene one and obtained attractive values: ΦF = 56% for CN and 51% for NHBoc vs. 9,10-diphenylanthracene and ΦF = 78% for CN and 68% for NHBoc vs. fluorene, of the same order as other conjugated polyfluorenes. Similar polymers provided higher values for both CN (ref. 9) and NHBoc (ref. 11) related systems, a disparity which cannot be clearly ascribed to different molar masses or just to uneven measuring methods.
Experimental
All commercially available reagents were purchased from ABCR, Acros, Aldrich or TCI and used without further purification unless otherwise specified. Solvents were purified and dried – if necessary – according to standard procedures.All reactions and manipulations involving air-sensitive compounds were carried out under nitrogen by using standard Schlenk techniques and dry, oxygen-free solvents. The Pd[P(p-tolyl)3]3 and Pd0(PPh3)4 catalysts used for SPC were prepared according to literature procedures and stored in inert atmosphere (glovebox). All involved experimental details and procedures are presented in the ESI.†
Solution NMR spectroscopy was performed with a Bruker AM (1H: 300.23, 500 MHz) at room temperature using deuterated chloroform (CDCl3), dimethyl sulfoxide ((CD3)2SO) and 1,1,2,2-tetrachloethane (C2D2Cl4) as solvents. The solvent signal was used as internal standard of the chemical shift (CDCl3: δH 7.26 ppm; (CD3)2SO: δH 2.50 ppm; C2D2Cl4: δH 6.0 ppm).
Dimethylformamide (DMF) analytical gel permeation chromatography (GPC) measurements were carried out using a PL-GPC 220 instrument with a 2× PL-Gel Mix-B LS column set equipped with RI (refractive index), viscosity and LS (light scattering with 15° and 90° angle) detectors (DMF + 1 g L−1 LiBr as eluent at 80 °C). Universal calibration was done using polystyrene standards of known molecular weight.
High-resolution mass spectrometry (HRMS) analyses were realized by the MS service in the laboratory of organic chemistry at ETH Zurich using an electron spray ionization (ESI) MS spectrometer with a quadrupole time-of-flight tandem mass analyzer (Q-TOF) (Bruker Daltonics maXis) or electron-impact ionization (EI) (Micromass AutoSpec-Ultima).
MALDI-TOF mass spectra were recorded using a Bruker UltraFlex II MALDI-TOF mass spectrometer (Bremen, Germany) equipped with a N2 smartbeam laser (λ = 337 nm, 150 μJ, 3 ns) operating at a pulse rate of 100 Hz. For MALDI-TOF MS analyses, the ions were accelerated with pulsed ion extraction (PIE) by a voltage of 25 kV. The analyzer was operated in reflection mode and the ions were detected using a microchannel plate detector. A total of 500–1000 shots were accumulated for each mass spectrum. Trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) with AgOTf was used as matrix material.
Mass spectrum of low molecular weight polyfluorene was simulated by means of mMass Open Source Mass Spectrometry Tool software.
TGA was carried out with a TGA Q500 (TA Instruments, USA) under N2 atmosphere in a platinum pan, at a scan rate of 10 °C min−1 for samples with a mass of between 4 and 6 mg.
Infrared spectroscopic analyses were carried out using an attenuated total reflection (ATR) Fourier transform infrared (FTIR) spectrometer (Bruker Optics Alpha system with a built-in diamond ATR), with OPUS 6 from Bruker as software. The data represent the average of 24 scans in the wavenumber range between 4000 and 500 cm−1 at a resolution of 1.42 cm−1.
UV/Vis absorption spectra were recorded in DMF solutions at room temperature with a Cary 1E spectrophotometer from Varian (Australia) using a quartz cell with a path length of 1 cm.
Emission spectra were recorded in DMF solutions at room temperature with a Spex Fluorolog 2 spectrophotometer from Jobin Yvon (United Kingdom) using a quartz cell with a path length of 1 cm. Fluorescence quantum yields were determined by standard procedures.
Conclusions
The present work shows that high molar mass polyfluorenes with nitrile and Boc-protected amine groups in the side chains are accessible through the SPC protocol despite contrary reports in the literature according to which either low molar products were obtained or no polymers at all. Using carefully purified monomers and optimized polymerization conditions proved to be the key to this success. The product polymers were obtained reproducibly on the gram scale and as typical polyfluorene-based materials. The importance of this finding extends beyond the field of polyfluorenes since to the best of our knowledge there are no reports on other SPC products with such functionalities that would have high molar masses. It may thus be advisable to re-visit some of the work published earlier, as conjugated polymers with lateral functional groups are attractive for modifications, e.g. resulting in insulation or protection against collisional quenching of fluorescence. To our surprise, despite the similarities between the two polymeric systems reported here, the best results were obtained by employing two completely different catalysts, PdII(dppf)Cl2 and Pd0[P(p-tolyl)3]3. Although the choice of the best catalytic system is still a matter of intuition and better results could possibly be obtained by exploring other catalyst precursors, our goal was to investigate the effect of the ones most often used in the mainstream literature regarding SPC, a statement also pertinent to the solvents.The issues encountered during SPC led to a thorough investigation of the oligomeric species obtained as side products. This resulted in valuable mechanistic insights showing ligand scrambling as the major side reaction, which requires attention if further optimization is intended.
While we believe to have reached a satisfactory state, there is still room for further research and engineering regarding the challenge of achieving higher molecular weights and yields for the envisaged polymeric structures.
Acknowledgements
The financial support by the SCIEX – NMSch Programme (project code 11.136) is gratefully acknowledged.Notes and references
- (a) N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437 CrossRef; (b) N. Miyaura and A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 866 RSC; (c) N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513 CrossRef CAS.
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6723 Search PubMed; (b) M. García-Melchor, A. A. C. Braga, A. Lledós, G. Ujaque and F. Maseras, Acc. Chem. Res., 2013, 46, 2626 CrossRef PubMed; (c) A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC.
- (a) M. Rehahn, A. D. Schlüter, G. Wegner and W. J. Feast, Polymer, 1989, 30, 1060 CrossRef CAS; (b) M. Rehahn, A. D. Schlüter and G. Wegner, Makromol. Chem., 1990, 191, 1991 CrossRef CAS.
- (a) A. D. Schlüter and G. Wegner, Acta Polym., 1993, 44, 59 CrossRef; (b) J. Sakamoto, M. Rehahn, G. Wegner and A. D. Schlüter, Macromol. Rapid Commun., 2009, 30, 653 CrossRef CAS PubMed; (c) J. Sakamoto and A. D. Schlüter, in Synthesis of Polymers: New Structures and Methods, ed. A. D. Schlüter, C. J. Hawker and J. Sakamoto, Wiley-VCH, Weinheim, 1st edn, 2012, vol. 2, ch. 2, pp. 627–676 Search PubMed; (d) Y. Segawa, T. Higashihara and M. Ueda, Polym. Chem., 2013, 4, 1208 RSC.
- (a) J. Murage, J. W. Eddy, J. R. Zimbalist, T. B. McIntyre, Z. R. Wagner and F. E. Goodson, Macromolecules, 2008, 41, 7330 CrossRef CAS; (b) R. Molina, S. Gomez-Ruiz, F. Montilla, A. Salinas-Castillo, S. Fernandez-Arroyo, M. del Mar Ramos, V. Micol and R. Mallavia, Macromolecules, 2009, 42, 5471 CrossRef CAS; (c) T.-X. Zhang and Z. Li, Comput. Theor. Chem., 2013, 1016, 28 CrossRef CAS; (d) H. Komber, S. Mullers, F. Lombeck, A. Held, M. Walter and M. Sommer, Polym. Chem., 2014, 5, 443 RSC.
- (a) M. Bernius, M. Inbasekaran, E. Woo, W. Wu and L. Wijkowski, J. Mater. Sci.: Mater. Electron., 2000, 11, 111 CrossRef CAS; (b) A. C. Grimsdale and K. Müllen, Adv. Polym. Sci., 2006, 199, 1 CrossRef CAS; (c) P. Chen, G. Yang, T. Liu, T. Li, M. Wang and W. Huang, Polym. Int., 2006, 55, 473 CrossRef CAS; (d) S.-A. Chen, H.-H. Lu and C.-W. Huang, Adv. Polym. Sci., 2008, 212, 49 CAS; (e) X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832 CrossRef CAS.
- (a) J. Frahn, B. Karakaya, A. Schäfer and A. D. Schlüter, Tetrahedron, 1997, 53, 15459 CrossRef CAS; (b) F. E. Goodson, T. I. Wallow and B. M. Novak, Macromolecules, 1998, 31, 2047 CrossRef CAS; (c) K. Treacher, P. Stossel, H. Spreitzer, H. Becker and A. Falcou, US Pat. 6,956,095B2, 2005; (d) A. Yokoyama, H. Suzuki, Y. Kubota, K. Ohuchi, H. Higashimura and T. Yokozawa, J. Am. Chem. Soc., 2007, 129, 7236 CrossRef CAS PubMed; (e) R. Tkachov, V. Senkovskyy, T. Beryozkina, K. Boyko, V. Bakulev, A. Lederer, K. Sahre, B. Voit and A. Kiriy, Angew. Chem., Int. Ed., 2014, 53, 2402 CrossRef CAS PubMed; (f) M. Liu, Y. Chen, C. Zhang, C. Li, W. Li and Z. Bo, Polym. Chem., 2013, 4, 895 RSC.
- B.-S. Kim, C.-G. Kim, J.-J. Oh, M.-S. Kim, G.-W. Kim and D.-K. Park, Macromol. Res., 2006, 14, 343 CrossRef CAS.
- R. Mallavia, F. Montilla, I. Pastor, P. Velasquez, B. Arredondo, A. L. Alvarez and C. R. Mateo, Macromolecules, 2005, 38, 3185 CrossRef CAS.
- M. Yu, Y. Tang, F. He, S. Wang, D. Zheng, Y. Li and D. Zhu, Macromol. Rapid Commun., 2006, 27, 1739 CrossRef CAS.
- B. Zhu, Y. Han, M. Sun and Z. Bo, Macromolecules, 2007, 40, 4494 CrossRef CAS.
- Z.-S. Guo, J. Pei, Z.-L. Zhou, L. Zhao, G. Gibson, S. Lam and J. Brug, Polymer, 2009, 50, 4794 CrossRef CAS.
- A. C. Grimsdale and K. Müllen, Adv. Polym. Sci., 2008, 212, 1 CAS.
- (a) A. D. Schlüter, Top. Curr. Chem., 2005, 245, 151 Search PubMed; (b) J. I. Paez, M. Martinelli, V. Brunetti and M. C. Strumia, Polymers, 2012, 4, 355 CrossRef; (c) O. V. Borisov, A. A. Polotsky, O. V. Rud, E. B. Zhulina, F. A. M. Leermakerse and T. M. Birshtein, Soft Matter, 2014, 10, 2093 RSC.
- M. Inbasekaran, W. Wu and E. P. Woo, WO 9920675, 1999.
- A. Balamurugan, M. L. P. Reddy and M. Jayakannan, J. Phys. Chem. B, 2011, 115, 10789 CrossRef CAS PubMed.
- B. Hohl, L. Bertschi, X. Zhang, A. D. Schlüter and J. Sakamoto, Macromolecules, 2012, 45, 5418 CrossRef CAS.
- (a) R. Kandre, K. Feldman, H. E. H. Meijer, P. Smith and A. D. Schlüter, Angew. Chem., Int. Ed., 2007, 46, 4956 CrossRef CAS PubMed; (b) S. Jakob, A. Moreno, X. Zhang, L. Bertschi, P. Smith, A. D. Schlüter and J. Sakamoto, Macromolecules, 2010, 43, 7916 CrossRef CAS.
- F. Samperi, S. Battiato, C. Puglisi, U. Giovanella, R. Mendichi and S. Destri, Macromolecules, 2012, 45, 1811 CrossRef CAS.
- (a) S. K. Weber, F. Galbrecht and U. Scherf, Org. Lett., 2006, 8, 4039 CrossRef CAS PubMed; (b) E. Zhou, J. Cong, S. Yamakawa, Q. Wei, M. Nakamura, K. Tajima, C. Yang and K. Hashimoto, Macromolecules, 2010, 43, 2873 CrossRef CAS.
- A. D. Schlüter, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1533 CrossRef.
- F. Huang, H. Wu, D. Wang, W. Yang and Y. Cao, Chem. Mater., 2004, 16, 708 CrossRef CAS.
- A. Monkman, C. Rothe, S. King and F. Dias, Adv. Polym. Sci., 2008, 212, 187 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental details and procedures, including NMR and HR-MS data, a simulated MALDI-TOF mass spectrum of the lower mass fraction, and fluorescence spectra of both polymers at different concentrations in DMF solutions. See DOI: 10.1039/c4ra09710f |
| This journal is © The Royal Society of Chemistry 2014 |