Rapid synthesis of a flower-like ZnO/rGO/Ag micro/nano-composite with enhanced photocatalytic performance by a one-step microwave method†
Abstract
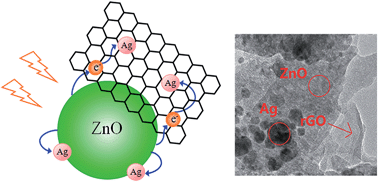
Flower-like ZnO/rGO/Ag micro/nano-composites (MNCs) have been engineered by a one-step microwave technique using graphene oxide, AgNO3 and Zn(CH3COO)2 as raw materials without adding any external toxic reagent. This is a facile and rapid process requiring only low power microwave irradiation (120 W). Various characterization results showed that the flower-like ZnO/rGO/Ag MNCs consisted of a ZnO nanosheet and Ag nanoparticles and reduced graphene oxide (rGO) deposited on ZnO nanosheet surface. The composite shows an enhanced and faster ultraviolet and simulated daylight photocatalytic property, i.e. 92.73% and 70.43% degradation of methyl orange in 20 minutes as compared to the values of 70.91% and 60.82%, 55.48% and 50.61% by bare ZnO and rGO/ZnO, respectively. The enhanced photocatalytic property is attributed to an efficient charge transfer process from ZnO to both Ag and rGO. This method would be beneficial for synthesizing efficient ZnO-based ternary photocatalysts with a combination of metal and rGO.

 Please wait while we load your content...
Please wait while we load your content...