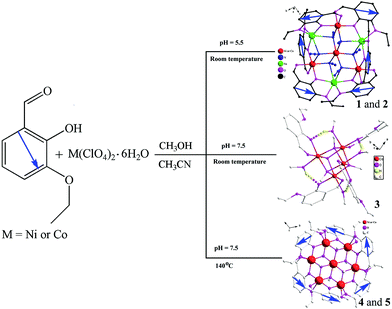
Structural variation from heterometallic heptanuclear or heptanuclear to cubane clusters based on 2-hydroxy-3-ethoxy-benzaldehyde: effects of pH and temperature†
Shu-Hua Zhang*a,
Ru-Xia Zhaoa,
Gui Lia,
Hai-Yang Zhanga,
Chun-Lian Zhanga and
Gilles Muller*b
aCollege of Chemistry and Bioengineering (Guangxi Key Laboratory of Environmental Friendly Electromagnetic Chemistry Function Materials), Guilin University of Technology, Guilin, People's Republic of China 541004. E-mail: zsh720108@163.com; Fax: +86 773 589 6839; Tel: +86 773 589 6839
bDepartment of Chemistry, San José State University, One Washington Square, San José, CA 95192-0101, USA. E-mail: gilles.muller@sjsu.edu; Fax: +1 408 924 4945; Tel: +1 408 924 5000
First published on 9th October 2014
Abstract
Five new Hheb complexes, HN(C2H5)3·[M4Na3(heb)6(μ3-N3)6], (M = Ni (1), and Co (2), [Co4(heb)4(μ3-OCH3)4(μ1-HOCH3)4]·(H2O)2 (3), [M7(heb)6(μ3-OCH3)6]·(ClO4)2 (M = Ni (4), and Co (5), Hheb = 2-hydroxy-3-ethoxy-benzaldehyde), were synthesized by reaction of hexahydrate perchlorate salt, Hheb, and NaN3 under different temperature and pH conditions. Careful investigation of the effect of the reaction temperature and pH resulted in a series of compounds with different compositions and nuclearities. The diverse compounds obtained illustrate the marked sensitivity of the structural chemistry of Co- or Ni-Hheb ligand-like systems to synthesis conditions. Complexes 1 and 2, which are heterometallic heptanuclear anion [M4Na3(heb)6(N3)6]− clusters, are formed at a pH of 5.5 and at room temperature. At a pH of 7.5 and at room temperature, a neutral molecular cubane cluster, namely 3, is formed with a lower nuclearity. Further increase of the reaction temperature to 140 °C at the same pH resulted in formation of two heptanuclear cation [M7(heb)6(μ3-OCH3)6]2+ clusters, 4 and 5. The results show that the pH and reaction temperatures play a key role in the structural control of the self-assembly process. Interestingly, heterometallic heptanuclear anion [M4Na3(heb)6(N3)6]− clusters have never been reported for the family of μ3-N3− or μ3-O-bridged heptanuclear clusters. The magnetic properties of 1–5 were investigated and are discussed in detail.
1. Introduction
The rational design and synthesis of polynuclear complexes have undergone tremendous development because they have fascinating structures and functional applications as optical, electronic, catalytic, fluorescent, and magnetic materials.1–4 Particular interest has focused on the development of single molecule magnets (SMM).5 An effective and facile approach for the synthesis of such complexes is still dependent on suitable choice of well-designed organic ligands as bridges or terminal groups with metal ions or metal clusters as nodes. Although systematic, accurate prediction and total design of crystal structure are not yet possible, efforts have been made to understand and control certain reaction parameters such as the metal-to-ligand molar ratio, the ligand denticity, the type of metal ions, the organic guest molecules, the presence of solvent molecules, the pH of the solution, the counter-ions, and the reaction temperatures that play crucial roles in the structure formation processes.2e,f,6,7 For example, Li et al. reported that pH has a crucial role in structural formation of 3,5-pyrazoledicarboxylic acid systems.6c,d Jacobson et al. reported that reaction temperature and pH influence the coordination modes of the 1,4-benzenedicarboxylate ligand.6e In addition, our investigation of systems involving 2-hydroxy-benzaldehyde ramification (HL) ligands or (1H-benzimidazol-2-yl)-methanol ramification ligands illustrated that steric hindrance of the ligands has a crucial role in structure formation.2f,3f As part of our ongoing interest in understanding the effects of temperature and pH on the assembly of clusters, we have recently succeeded in selective synthesis of five clusters by controlling reaction temperature and pH. Details of the synthesis and characterization of these complexes with multidentate ligand 2-hydroxy-3-ethoxy-benzaldehyde (Hheb, Scheme 1) are described in this paper.This ligand has three oxygen atoms from the phenoxo, aldehyde and C2H5O− groups, and has potential as use as a linker for construction of interesting coordination polymers with abundant hydrogen bonds.2f,5a,8 It is worth noting that the ligand 2-hydroxy-3-methoxy-benzaldehyde has been reported to possess four potential coordination modes: μ5:η2:η2:η1,9 μ2:η1:η1,5a,10 μ4:η1:η2:η1,2f,11 and μ6:η3:η2:η1,12 whereas its analogue, 2-hydroxy-3-ethoxy-benzaldehyde (Hheb) has been reported to possess only three coordination modes, μ2:η1:η1,13 μ4:η1:η2:η1,1e and μ5:η2:η2:η1.14 As a result, it was decided to investigate the coordination mode of the multidentate Hheb ligand with the objective of constructing new clusters by controlling temperature and pH during the preparation of these compounds.
2. Experimental
Materials and instrumentation
All chemicals were commercially available and used as received without further purification. Elemental analyses (CHN) were performed using an Elemental Vario-EL CHN elemental analyzer. FT-IR spectra were recorded from KBr pellets in the range of 4000–400 cm−1 on a Bio-Rad FTS-7 spectrometer. The X-ray crystal structures were determined by single-crystal X-ray diffraction using the SHELXL crystallographic software for molecular structures. The PXRDs of 1–5 were determined by Rigaku D/max 2500v/pc. Magnetization measurements were carried out with a Quantum Design MPMS-XL7 SQUID to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe for 1–5 ((HN(C2H5)3·[M4Na3(heb)6(μ3-N3)6] with M = Ni (1) and Co (2), [Co4(heb)4(μ3-OCH3)4(μ1-HOCH3)4]·(H2O)2 (3), [M7(heb)6(μ3-OCH3)6]·(ClO4)2 with M = Ni (4) and Co (5)). It must be mentioned that the χMT vs. T curves for all complexes of interest (except 3) cannot be fitted by the Magpack method.
000 Oe for 1–5 ((HN(C2H5)3·[M4Na3(heb)6(μ3-N3)6] with M = Ni (1) and Co (2), [Co4(heb)4(μ3-OCH3)4(μ1-HOCH3)4]·(H2O)2 (3), [M7(heb)6(μ3-OCH3)6]·(ClO4)2 with M = Ni (4) and Co (5)). It must be mentioned that the χMT vs. T curves for all complexes of interest (except 3) cannot be fitted by the Magpack method.
Syntheses
Caution: perchlorate salts and azide of metal complexes with organic ligands are potentially explosive.
Crystal structure determination
The diffraction data were collected on an Agilent G8910A CCD diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å), using the ω–θ scan mode in the ranges 2.97° ≤ θ ≤ 25.10° (1), 2.88° ≤ θ ≤ 25.01° (2), 2.89° ≤ θ ≤ 25.10° (3), 3.49° ≤ θ ≤ 25.08° (4), and 3.17° ≤ θ ≤ 25.05° (5). Raw frame data were integrated with the SAINT program. The structures were solved by direct methods using SHELXS-97 and refined by full-matrix least-squares on F2 using SHELXS-97.15 An empirical absorption correction was applied with the program SADABS.15 All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were positioned geometrically and refined as riding. Calculations and graphics were performed with SHELXTL.15 The anisotropic displacement parameters of atoms C55–C60 in 1 and 2 were restrained to be identical with a standard uncertainty of 0.01 Å2. The structures contain solvent accessible voids of 250 and 235 Å3 for 1 and 2, respectively. The highest peaks with 1.340 e Å−3 of 3 and 1.187 e Å−3 of 5 in the residual electron density are located 0.09 Å from atom H6A and 0.75 Å from atom H8B, respectively. The crystallographic details are provided in Table 1. Selected bond distances and angles for 1–5 are listed in Tables S1–S4† and hydrogen bond lengths (Å) and angles (°) in Complexes 1–3 are listed in Tables S5–S7.†| Complexes | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| a R1 = Σ‖Fo| − |Fc‖/Σ|Fo|.b wR2 = [Σw(|Fo2| − |Fc2|)2/Σw(|Fo2|)2]1/2. | |||||
| Formula | C60H70N19Na3Ni4O18 | C60H70N19Na3Co4O18 | C44H68Co4O22 | C60H72Cl2Ni7O32 | C60H72Cl2Co7O32 |
| Mr | 1649.08 | 1650.04 | 1184.70 | 1786.95 | 1788.59 |
| Crystal size (mm) | 0.25 × 0.10 × 0.06 | 0.22 × 0.19 × 0.16 | 0.12 × 0.10 × 0.08 | 0.18 × 0.16 × 0.15 | 0.16 × 0.14 × 0.12 |
| Crystal system | Monoclinic | Monoclinic | Tetragonal | Hexagonal | Hexagonal |
| Space group | P21/n | P21/n | P4/ncc | P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
| a (Å) | 13.200 (1) | 13.245 (1) | 17.0166 (4) | 14.649 (1) | 14.818 (1) |
| b (Å) | 26.664 (1) | 26.530 (1) | 17.0166 (4) | 14.649 (1) | 14.818 (1) |
| c (Å) | 22.120 (1) | 22.159 (1) | 18.7995 (8) | 9.663 (1) | 9.391 (1) |
| α (°) | 90 | 90 | 90 | 90 | 90 |
| β (°) | 96.526 (3) | 96.648 (3) | 90 | 90 | 90 |
| γ (°) | 90 | 90 | 90 | 120 | 120 |
| V (Å3) | 7735.1 (5) | 7734.0 (4) | 5443.7 (3) | 1795.7 (2) | 1785.8 (3) |
| F(000) | 3412 | 3396 | 2464 | 918 | 911 |
| Z | 4 | 4 | 4 | 1 | 1 |
| Dc (g cm−3) | 1.417 | 1.418 | 1.446 | 1.652 | 1.663 |
| μ (mm−1) | 1.051 | 0.935 | 1.271 | 1.956 | 1.747 |
| θ range (°) | 2.97–25.10 | 2.88–25.01 | 2.89–25.10 | 3.49–25.08 | 3.17–25.05 |
| Ref. meas./indep. | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163, 13 163, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550, 13 550, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 561 561 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303, 2432 303, 2432 |
3860, 2128 | 3653, 2111 |
| Obs. ref. [I > 2σ(I)] | 8437 | 9059 | 1872 | 1602 | 1602 |
| Rint | 0.0400 | 0.0565 | 0.0296 | 0.0210 | 0.0296 |
| R1 [I ≥ 2σ (I)]a | 0.0549 | 0.0716 | 0.0543 | 0.0547 | 0.0566 |
| wR2 (all data)b | 0.1797 | 0.2229 | 0.2013 | 0.1865 | 0.1657 |
| Goof | 1.002 | 1.070 | 1.009 | 1.008 | 1.008 |
| Δρ (max, min) (e Å−3) | 0.707, −0.459 | 0.807, −0.605 | 1.340, −0.601 | 0.830, −0.577 | 1.187, −0.677 |
3. Results and discussion
Description of the crystal structures
As shown in Fig. S2,† the different cubes present in the cluster are well separated from each other by the heb ligands and methoxy groups resulting in cent–cent distances of adjacent cubanes of 9.40 Å in the c direction and 12.03 Å in the ab plan of intramolecular hydrogen bonds. Moreover, the nearest intercluster Co⋯Co distances are 9.464 Å in the ab plan and 7.41 Å in the c direction.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , will be discussed in detail. Its unit cell contains one disc-like heptanuclear nickel cluster [Ni7(heb)6(μ3-OCH3)6]2+ and two counter anions ClO4−, which lie on a site with a threefold symmetry (Fig. 3). In addition, six μ2-phenoxo groups bridge the six nickel ions on the rim, which are linked to the central nickel ion through six μ3-OCH3 bridging groups. These latter groups act as the ‘bottom’ segment in the formed disc-like structure. The coordination geometry of the central nickel ion (Ni2) can be described as a slightly distorted octahedron with bond lengths of 2.077 (3) Å (Ni2–O4) and O–Ni2–O angles in the range of 82.2 (2)–97.8 (2)° (cis-angles), whereas all of the trans-angles have a value of 180.0°. Each nickel ion on the rim (Ni1) adopts a NiO6 configuration as Ni1 is coordinated by four O atoms from two different heb ligands and two O(O4, O4b) atoms from two methoxy groups resulting in the formation of a slightly distorted octahedral geometry (Ni1–O1, 1.990 (4); Ni1–O1a, 1.997 (4); Ni1–O2a, 2.029 (5); Ni1–O4b, 2.045 (4); Ni1–O4; 2.076 (4) and Ni1–O3, 2.345 (4) Å; symmetry code: (a) x − y, x, 2 − z and (b) y − x, − x, 2 + z). The O–Ni1–O angles lie in the range of 72.4 (2)–104.7 (2)° and 151.9 (2)–172.8 (1)° for the cis- and trans-angles, respectively. Additionally, the magnetic exchange angles of Ni–O–Ni are in the range of 96.8 (2)–102.6 (2)°. The assignment of the metal ions on the rim (Ni1) and at the center (Ni2) of the structure was based on the valence sum calculation. The heb anionic ligands, which bridge the peripheral NiII centers, display a μ2:η1:η2:η1 coordination mode (Scheme 2c) and lie alternately above and below the {NiII} plane. Moreover, the packing mode for 4 and 5 can be described as AAA along the c axis as shown in the packing diagram of 4 (Fig. S3†). The disc-like Ni7 units are well separated from each other by heb ligands, methoxy groups and ClO4− ions, thus resulting in center distances between adjacent clusters of 14.65 Å and 14.82 Å in the ab plane, and 9.66 and 9.39 Å in the c direction for 4 and 5, respectively (Fig. S3†). On the other hand, the core of the cluster can be described as an almost planar {Ni6} moiety (a ±0.1143 Å deviation from the average plane), which is composed of [Ni1] and its five centrosymmetric equivalent Ni atoms, with the central nickel ion (Ni2) lying on a site with a threefold symmetry. Although the structural motif belongs to the expanding {M7} family,17–21 it is worth mentioning that only a few heptanuclear clusters have been reported so far.17–21 More importantly, to the best of our knowledge, 5 is the first example of a 2-hydroxy-3-ethoxy-benzaldehyde cobalt cluster.
, will be discussed in detail. Its unit cell contains one disc-like heptanuclear nickel cluster [Ni7(heb)6(μ3-OCH3)6]2+ and two counter anions ClO4−, which lie on a site with a threefold symmetry (Fig. 3). In addition, six μ2-phenoxo groups bridge the six nickel ions on the rim, which are linked to the central nickel ion through six μ3-OCH3 bridging groups. These latter groups act as the ‘bottom’ segment in the formed disc-like structure. The coordination geometry of the central nickel ion (Ni2) can be described as a slightly distorted octahedron with bond lengths of 2.077 (3) Å (Ni2–O4) and O–Ni2–O angles in the range of 82.2 (2)–97.8 (2)° (cis-angles), whereas all of the trans-angles have a value of 180.0°. Each nickel ion on the rim (Ni1) adopts a NiO6 configuration as Ni1 is coordinated by four O atoms from two different heb ligands and two O(O4, O4b) atoms from two methoxy groups resulting in the formation of a slightly distorted octahedral geometry (Ni1–O1, 1.990 (4); Ni1–O1a, 1.997 (4); Ni1–O2a, 2.029 (5); Ni1–O4b, 2.045 (4); Ni1–O4; 2.076 (4) and Ni1–O3, 2.345 (4) Å; symmetry code: (a) x − y, x, 2 − z and (b) y − x, − x, 2 + z). The O–Ni1–O angles lie in the range of 72.4 (2)–104.7 (2)° and 151.9 (2)–172.8 (1)° for the cis- and trans-angles, respectively. Additionally, the magnetic exchange angles of Ni–O–Ni are in the range of 96.8 (2)–102.6 (2)°. The assignment of the metal ions on the rim (Ni1) and at the center (Ni2) of the structure was based on the valence sum calculation. The heb anionic ligands, which bridge the peripheral NiII centers, display a μ2:η1:η2:η1 coordination mode (Scheme 2c) and lie alternately above and below the {NiII} plane. Moreover, the packing mode for 4 and 5 can be described as AAA along the c axis as shown in the packing diagram of 4 (Fig. S3†). The disc-like Ni7 units are well separated from each other by heb ligands, methoxy groups and ClO4− ions, thus resulting in center distances between adjacent clusters of 14.65 Å and 14.82 Å in the ab plane, and 9.66 and 9.39 Å in the c direction for 4 and 5, respectively (Fig. S3†). On the other hand, the core of the cluster can be described as an almost planar {Ni6} moiety (a ±0.1143 Å deviation from the average plane), which is composed of [Ni1] and its five centrosymmetric equivalent Ni atoms, with the central nickel ion (Ni2) lying on a site with a threefold symmetry. Although the structural motif belongs to the expanding {M7} family,17–21 it is worth mentioning that only a few heptanuclear clusters have been reported so far.17–21 More importantly, to the best of our knowledge, 5 is the first example of a 2-hydroxy-3-ethoxy-benzaldehyde cobalt cluster.
 | ||
| Fig. 3 The cation structures of [M7(heb)6(μ3-OCH3)6]2+ (M = Ni (4), and Co (5)). All H-atoms are omitted for clarity. | ||
Structural and synthetic aspects
Our aim was to investigate the effects of the temperature and pH on self-assembly of supramolecules and clusters. Co(II), Ni(II), and Hheb were selected as starting materials. The synthetic strategy for the Hheb system is depicted in Scheme 2. Complexes 1–3 were obtained under similar conditions with the exception of the pH. At first, we carried out the complex synthesis at a pH of 5.5 and at room temperature, and compounds 1 and 2 with heterometallic heptanuclear anion [M4Na3(heb)6(N3)6]− clusters were obtained. When the pH of the reaction was raised to 7.5, complex 3 with a cubane cluster was obtained. It is interesting to note that the anionic group N3− is not present in 3, unlike as seen in 1 and 2. On the basis of the synthetic conditions used to prepare 1–3, it can be concluded that the pH is most likely responsible for the structural differences observed between 1, 2, and 3. In addition, to affect the deprotonation of the organic ligands used in the formation of the three complexes, an increase in the pH conditions also results in the presence of solvent anionic species such as CH3O− at higher pH (7.5). As a result, the methanol molecules exist in the form of CH3O− anionic species, which can be coordinated as μ3-OCH3 in 3 at a pH of 7.5. On the other hand, the methanol molecules only function as solvent species in the formation of 1 and 2 when the pH is maintained at 5.5. It should be mentioned that a different single crystal growth time was used for the formation of the three complexes, which could also affect the type of systems obtained. The single crystal growth time was about 30 minutes for 1 and 2, whereas a much longer time was required for 3 (3 days).In addition to the pH effect, temperature also has an influence on the type of the compounds obtained. Increasing the temperature from room temperature to 140 °C resulted in formation of two heptanuclear cationic [M7(heb)6(μ3-OCH3)6]2+ clusters 4 and 5 when the pH was adjusted with triethylamine to 7.5. In comparison with 3, which was synthesized at room temperature, the findings regarding 4 and 5 show that the solvothermal temperature also plays an important role in formation of different clusters (e.g., 1–5). According to the above discussion and considering the comparison of 1 and 2 with 3, we can conclude that a lower pH (5.5) is necessary for coordination of azide ions whereas a higher pH (7.5) is needed for the presence of CH3O− anions in the solution, which can then coordinate as μ3-OCH3 species in 3. At the same time, comparing 3 and 4 with 5, we can conclude that lower temperatures (room temperature) and higher temperatures (140 °C) may result in formation of lower and higher nuclear complexes, respectively. This is most likely because of an increase in the degree of condensation of metal polyhedra and the ligand coordination ability when the reaction temperature is increased.6e,7f,24
Furthermore, it was decided to investigate the influence of the bulky ethyl group on the formation of such self-assembly systems. As such, 2-hydroxy-3-methoxy-benzaldehyde (Hhmb) was used instead of 2-hydroxy-3-ethoxy-benzaldehyde under the same experimental conditions as 1 and 2. The two compounds obtained, which result in formation of two heterometallic [M2Na2(μ1,1,1-N3)2(hmb)4(CH3CN)2]·(CH3CN)2 (M = Ni (a),25 M = Co (b),26 Fig. S4†) clusters with two face-sharing cubes (each with one vertex missing), were different from those expected (HN(C2H5)3·[M4Na3(hmb)6(N3)6]). This is most likely because Hhmb and Hheb present different coordination abilities, resulting presumably from the steric hindrance of the RO groups, even if the phenolic hydroxyl, aldehyde, and RO-groups in the coordination sites of Hhmb and Hheb are similar.
Magnetic properties
The magnetic susceptibilities of 1–5 were measured from crushed single crystalline samples (the phase purities of 1–5 have been checked by PXRD patterns, see Fig. S5†), and variable-temperature direct-current (dc) magnetic susceptibility data were collected for 1–5 in the temperature range of 2–300 K under an applied field of 1000 Oe.Magnetic properties of 1 and 4
For complexes 1 and 4, spin–orbital coupling of Ni(II) ions gives rise to a value of the χMT product of 5.84 cm3 K mol−1 (1) and 9.13 cm3 K mol−1 (4) at room temperature (Fig. 4). This behavior suggests an orbital contribution of the distorted octahedral Ni(II) ions. For 1, this value is higher than the calculated spin-only value of 4.4 cm3 K mol−1 from four non-interaction high-spin Ni(II) ions assuming g = 2.2. The observed value of 1 is also slightly higher than those obtained for [Ni4(ROH)4L4] (H2L = salicylidene-2-ethanolamine; R = Me or Et) (∼5.1 cm3 K mol−1)27 and [Ni4(μ3-OMe) (MeOH)4L4] (H2L is 2-hydroxy-3-methoxybenzaldehyde) (∼5.45 cm3 K mol−1),5a but smaller than for [HN(C2H5)3]8·[Ni4(dchaa)4(N3)4]2 (dchaa is the anion of 3,5-dichloro-2-hydroxy-benzylaminoacetic acid) (∼6.54 cm3 K mol−1).22b The χMT value of 1 at room temperature lies in the range of other tetranuclear nickel clusters, which have χMT values between 5.1 and 6.61 cm3 K mol−1.5a,22b,28,29 Upon decreasing T, the χMT products of 1 and 4 gradually increase to maximum values of 14.67 and 10.13 cm3 K mol−1 at 35 K, and then smoothly fall to 5.7 and 7.47 cm3 K mol−1 at 2 K, respectively. Similar magnetic behaviors were observed for [Ni4(ROH)4L4],27 [Ni4(μ3-OMe)(MeOH)4L4],5a [Ni4(η1,μ3-N3)4(dbm)4(EtOH)4]·2C7H8 (Hdbm is dibenzoylmethane),28 and [Ni7(mmimp)6(CH3O)6]·(X)2.17c The sudden decrease of χMT is assigned to zero-field splitting in the ground state, Zeeman effects, or intercluster antiferromagnetic interactions at low temperatures.The temperature dependence of the reciprocal susceptibility χM−1 above 50 K follows the Curie–Weiss law [χ = C/(T − θ)] with Weiss and Curie constants of 39.34 (1) K and 5.18 (1) cm3 K mol−1 for 1 and 5.18 (1) K and 9.01 (1) cm3 K mol−1 for 4, respectively (Fig. S6a and d†). The larger positive Weiss constants suggest an intramolecular ferromagnetic interaction between adjacent Ni(II) ions through the μ3-N3 and μ3-O bridges in 1 and 4. For 1, the observation of a maximum of χMT at 35 K is indicative of an S = 4 ground state (with g = 2.25, χMT = 0.125g2ST(ST + 1) ≈ 12.66 cm3 K mol−1). As such, this pattern is compatible with moderate ferromagnetic coupling.29 Further evidence of ferromagnetic coupling between Ni(II) ions was observed in the variable-field magnetization curves plotted in Fig. S7a and d.† At low fields from 0 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, the magnetization of 1 and 4 sharply increases. For 1, above 10
000 Oe, the magnetization of 1 and 4 sharply increases. For 1, above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, the magnetization slowly increases and saturates at 8.85 NμB when a field of 50
000 Oe, the magnetization slowly increases and saturates at 8.85 NμB when a field of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe is achieved, which is consistent with M = gST = 2.22 × 4 = 8.88 NμB. For 4, above 10
000 Oe is achieved, which is consistent with M = gST = 2.22 × 4 = 8.88 NμB. For 4, above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, it slowly increases but does not saturate at 50
000 Oe, it slowly increases but does not saturate at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. Finally, AC susceptibility measurements were carried out in the 2–10 K range at frequencies of 10 Hz, 100 Hz, 300 Hz, and 997 Hz for 1 and 100, 997 Hz for 4 (Fig. S8a and d†). The results of these measurements confirmed that 1 and 4 do not behave as SMMs, which is corroborated by no out-of-phase ac signals being observed above 2 K.
000 Oe. Finally, AC susceptibility measurements were carried out in the 2–10 K range at frequencies of 10 Hz, 100 Hz, 300 Hz, and 997 Hz for 1 and 100, 997 Hz for 4 (Fig. S8a and d†). The results of these measurements confirmed that 1 and 4 do not behave as SMMs, which is corroborated by no out-of-phase ac signals being observed above 2 K.
Magnetic properties of 2, 3, and 5
As seen for 1, the complexes 2, 3, and 5 show that, at room temperature, the spin–orbital coupling of the Co(II) ions gives rise to χMT products of 12.53, 9.15, and 31.85 cm3 K mol−1 for 2, 3, and 5, respectively (Fig. 5). For 2 and 3, the obtained values are much higher than the calculated spin-only value of 7.5 cm3 K mol−1 from four non-interacting high-spin Co(II) ions, assuming g = 2.0.5a,30 On the other hand, 5 shows that the χMT value is much higher than the calculated spin-only value (13.1 cm3 K mol−1) of seven high-spin non-interacting Co(II) ions with the assumption that g is equal to 2.0.21h These findings can be explained by the orbital contribution to the magnetic moment of Co(II). For 2, with decreasing T, χMT gradually rises to a local maximum of 20.08 cm3 K mol−1 at 14 K before falling to 8.38 cm3 K mol−1 at 2 K. The characteristic pattern often observed for Co(II) complexes because of a strong orbital contribution, consisting of a significant decrease in the value of χMT as the temperature is lowered, is not seen for 2.31 This can be explained as follows: (i) the orbital contribution is partially quenched because of the distortions from the typical octahedral symmetry at the Co(II) centers, and (ii) there is an increase in χMT below 37 K because of the ferromagnetic exchange interactions between the Co(II) centers. This is consistent with similar magnetic behavior that was observed for a Co12 wheel.32 For 3, the χMT value slowly falls off on cooling to a value of 8.5 cm3 K mol−1 at 60 K, after which it rapidly decreases to 4.23 cm3 K mol−1 when a temperature of 2 K is achieved. This pattern most likely indicates the occurrence of a relatively weak antiferromagnetic intra-cluster interaction between the four Co(II) ions. For 5, the χMT value decreases gradually and reaches a minimum of 30.05 cm3 K mol−1 at 40 K (Fig. 5). In the range of 300–40 K, the magnetic properties of 5 mainly exhibit single-ion behavior of the Co(II) ion. Below 40 K, it can be suggested that the pattern observed (a slight increase of the χMT value up to a maximum of 31.37 cm3 K mol−1 at 14 K, and then a sharp decrease to 9.87 cm3 K mol−1 at 2 K) is the consequence of ferromagnetic coupling between the Co(II) ions offsetting the effect of the spin–orbital coupling, and thus compensating for the decrease in the χMT value. This is consistent with similar magnetic behavior observed for [Co7(bzp)6(N3)9(CH3O)3](ClO4)2·2H2O,33 [CoII4CoIII3(HL)6(NO3)3(H2O)3]2+ {H3L = H2NC(CH2OH)3},21c Co12 wheel,32 and [Co7(immp)6(CH3O)6](ClO4)2 (immp is 2-iminomethyl-6-methoxy-phenolic anion).2a | ||
| Fig. 5 Plots of χMT and χM vs. T measured in a 1000 Oe field for 2 (a), 3 (b), and 5 (c). The solid lines represent the best fits of data between 300 and 50 K as described in the text for 3. | ||
The temperature dependence of the reciprocal susceptibility χM−1 above 50 K follows the Curie–Weiss law [χ = C/(T − θ)] with Weiss and Curie constants of 5.00 (1) K and 12.56 (1) cm3 K mol−1, −4.29 (1) K and 9.33 (1) cm3 K mol−1, and −1.98 K and 32.39 cm3 K mol−1 for 2, 3, and 5, respectively (Fig. S6b, c and e†). Compared with 3 and 5, the larger positive Weiss constant observed for 2 also suggests an intramolecular ferromagnetic interaction between adjacent Co(II) ions through the μ3-N3 bridges, whereas the maximum of χMT at 14 K is indicative of an S = 6 ground state (with g = 2.00, χMT = 0.125g2ST(ST +1) ≈ 21 cm3 K mol−1). Similarly, the larger negative Weiss constant observed for 3 compared with 1 and 5 suggests dominant intramolecular antiferromagnetic interactions between adjacent Co(II) ions through the μ3-O bridges. Finally, the contribution of a spin–orbital interaction discussed earlier has also an effect on the ferromagnetic exchange occurring in 5 (it is diminished). As a result, a smaller negative value of the Weiss constant was obtained. Further evidence of ferromagnetic coupling between Co(II) ions in 2, 3, and 5 was observed in the variable-field magnetization curves plotted in Fig. S7b, c and e.† At low fields from 0 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, the magnetization sharply increases, whereas above 10
000 Oe, the magnetization sharply increases, whereas above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, the magnetization slowly increases but does not saturate at 50
000 Oe, the magnetization slowly increases but does not saturate at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe for 2 and 3. This is different from what was observed for 5 as above 10
000 Oe for 2 and 3. This is different from what was observed for 5 as above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe the magnetization slowly increases and then saturates to 23.40 NμB at 50
000 Oe the magnetization slowly increases and then saturates to 23.40 NμB at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, which is consistent with M = gST = 2.22 × 21/2 = 23.31 NμB. Furthermore, the AC susceptibility measurements that were carried out in the 2–10 K range at frequencies of 100 Hz and 997 Hz (for 2 and 5), and 10, 100, 300, 600, 997 Hz for 3 (Fig. S8b, c and e†) suggested that 2, 3, and 5 do not behave as SMMs as no out-of-phase ac signals were detected above 2 K.
000 Oe, which is consistent with M = gST = 2.22 × 21/2 = 23.31 NμB. Furthermore, the AC susceptibility measurements that were carried out in the 2–10 K range at frequencies of 100 Hz and 997 Hz (for 2 and 5), and 10, 100, 300, 600, 997 Hz for 3 (Fig. S8b, c and e†) suggested that 2, 3, and 5 do not behave as SMMs as no out-of-phase ac signals were detected above 2 K.
According to the cubic structure of the cluster, the magnetic exchange between Co(II) ions in the core of Co4O4 observed for 3 can be assessed using Van Vleck's equation (eqn (1)). This is based on Kambe's method, which requires the use of the isotropic spin Hamiltonian  and where J1 is the coupling constant between the Co(II) ions.
and where J1 is the coupling constant between the Co(II) ions.
 | (1) |
The best fitting in the temperature range from 300 to 50 K gave g = 2.21 and J1 = −0.87 (1) cm −1 with R = 1.9 × 10−4. The negative coupling constant indicates a relatively weak antiferromagnetic intracube Co(II) interaction, which is different from those observed in several related octahedral Co(II) complexes with similar cuboidal cores.5a,34
Conclusions
Five new polynuclear clusters have been synthesized by modulating the pH and reaction temperature conditions. The results show that these two factors play a key role in the structural control of the self-assembly process. Additionally, magnetic studies indicate that 1, 2, 4, and 5 display dominant ferromagnetic intracluster interactions, whereas 3 displays an antiferromagnetic interaction between Co(II) ions. Subsequent works will focus on construction of novel polymers using 2-hydroxy-3-ethoxy-benzaldehyde as a basic building unit.Acknowledgements
This work is financially supported by the National Nature Science Foundation of China (no. 21161006), Program for Excellent Talents in Guangxi Higher Education Institutions (Gui Jiao Ren [2012]41). G.M. thanks the Henry Dreyfus Teacher-Scholar Award for financial support.References
- (a) C. S. Liu, X. S. Shi, J. R. Li, J. J. Wang and X. H. Bu, Cryst. Growth Des., 2006, 6, 656–663 CrossRef CAS; (b) B. Zhao, H. L. Gao, X. Y. Chen, P. Cheng, W. Shi, D. Z. Liao, S. P. Yan and Z. H. Jiang, Chem.–Eur. J., 2006, 12, 149–158 CrossRef CAS PubMed; (c) S. Hu, F. Y. Yu, P. Zhang and D. R. Lin, Dalton Trans., 2013, 42, 7731–7740 RSC; (d) S. Hu, F. Y. Yu, P. Zhang and A. J. Zhou, Eur. J. Inorg. Chem., 2012, 3669–3673 CrossRef CAS; (e) S. H. Zhang, R. X. Zhao, H. P. Li, C. M. Ge, Q. P. Huang and H. H. Zou, J. Solid State Chem., 2014, 216, 30–35 CrossRef CAS.
- (a) S. H. Zhang, Y. Song, H. Liang and M. H. Zeng, CrystEngComm, 2009, 11, 865–872 RSC; (b) Y. Xiao, P. Huang and W. Wang, J. Clust. Sci., 2014 DOI:10.1007/s10876-014-0799-9; (c) B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin and C. H. Yan, Angew. Chem., Int. Ed., 2000, 39, 3644–3946 CrossRef CAS; (d) L. F. Ma, L. Y. Wang, Y. Y. Wang, S. R. Batten and J. G. Wang, Inorg. Chem., 2009, 48, 915–924 CrossRef CAS PubMed; (e) S. H. Zhang, L. F. Ma, H. H. Zou, Y. G. Wang, H. Liang and M. H. Zeng, Dalton Trans., 2011, 40, 11402–11409 RSC; (f) S. H. Zhang, N. Li, C. M. Ge, C. Feng and L. F. Ma, Dalton Trans., 2011, 40, 3000–3007 RSC.
- (a) R. Q. Zou, H. Sakurai and Q. Xu, Angew. Chem., Int. Ed., 2006, 45, 2542–2546 CrossRef CAS PubMed; (b) P. Li, H. M. Liu, X. G. Lei, X. Y. Huang, D. H. Olson, N. J. Turro and J. Li, Angew. Chem., Int. Ed., 2003, 42, 542–546 CrossRef PubMed; (c) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS PubMed; (d) S. Hu, P. Zhang, F. Y. Yu, M. X. Chen and D. R. Lin, Polyhedron, 2014, 67, 388–395 CrossRef CAS; (e) L. Yang, S.-H. Zhang, W. Wang, J.-J. Guo, Q. P. Huang, R.-X. Zhao, C.-L. Zhang and G. Muller, Polyhedron, 2014, 74, 49–56 CrossRef CAS.
- M. Fujita, A. Powell and C. Creutz, From the molecular to the nanoscale: synthesis, structure, and properties, Elsevier Ltd., Oxford, 2004, vol. 7, pp. 1–56 Search PubMed.
- (a) S. H. Zhang, Y. D. Zhang, H. H. Zou, J. J. Guo, H. P. Li, Y. Song and H. Liang, Inorg. Chim. Acta, 2013, 396, 119–125 CrossRef CAS; (b) E. Tancini, M. Mannini, P. Sainctavit, E. Otero, R. Sessoli and A. Cornia, Chem.–Eur. J., 2013, 19, 16902–16905 CrossRef CAS PubMed; (c) M. Dey and N. Gogoi, Angew. Chem., Int. Ed., 2013, 52, 12780–12782 CrossRef CAS PubMed; (d) D. Aravena and E. Ruiz, Inorg. Chem., 2013, 52, 13770–13778 CrossRef CAS PubMed; (e) F. Habib, G. Brunet, V. Vieru, I. Korobkov, L. F. Chibotaru and M. Murugesu, J. Am. Chem. Soc., 2013, 135, 13242–13245 CrossRef CAS PubMed; (f) A. Deb, T. T. Boron, III, M. Itou, Y. Sakurai, T. Mallah, V. L. Pecoraro and J. E. Penner-Hahn, J. Am. Chem. Soc., 2014, 136(13), 4889–4892 CrossRef CAS PubMed; (g) R. A. Layfield, Organometallics, 2014, 33(5), 1084–1099 CrossRef CAS.
- (a) C. B. Aakeröy, J. Chem. Soc., Chem. Commun., 1998, 1067–1068 RSC; (b) V. A. Russell, C. C. Evans, W. Li and M. D. Ward, Science, 1997, 276, 575–579 CrossRef CAS PubMed; (c) L. Pan, X. Y. Huang and J. Li, J. Solid State Chem., 2000, 152, 236–246 CrossRef CAS; (d) L. Pan, T. Frydel, M. B. Sander, X. Y. Huang and J. Li, Inorg. Chem., 2001, 40, 1271–1283 CrossRef CAS PubMed; (e) Y. B. Go, X. Q. Wang, E. V. Anokhina and A. J. Jacobson, Inorg. Chem., 2005, 44, 8265–8271 CrossRef CAS PubMed; (f) Y. V. Kokunov and Y. E. Gorbunova, Russ. J. Inorg. Chem., 2007, 52, 1530–1535 CrossRef; (g) M. Sarkar, V. Bertolasi and D. Ray, Eur. J. Inorg. Chem., 2010, 2530–2536 CrossRef CAS.
- (a) S. K. Ghosh, G. Savitha and P. K. Bharadwaj, Inorg. Chem., 2004, 43, 5495–5497 CrossRef CAS PubMed; (b) J. W. Cheng, S. T. Zheng and G. Y. Yang, Dalton Trans., 2007, 4059–4066 RSC; (c) Y. Q. Huang, Z. L. Shen, T. Okamura, Y. Wang, X. F. Wang, W. Y. Sun, J. Q. Yu and N. Ueyama, Dalton Trans., 2008, 204–213 RSC; (d) M. Du, X. J. Jiang and X. J. Zhao, Inorg. Chem., 2006, 45, 3998–4006 CrossRef CAS PubMed; (e) P. M. Forster, N. Stock and A. K. Cheetham, Angew. Chem., Int. Ed., 2005, 44, 7608–7611 CrossRef CAS PubMed; (f) P. M. Foster, A. R. Burbank, C. Livage, G. Ferey and A. K. Cheetham, Chem. Commun., 2004, 368–369 RSC; (g) M. L. Tong, S. Kitagawa, H. C. Chang and M. Ohba, Chem. Commun., 2004, 418–419 RSC; (h) P. Mahata, A. Sundaresan and S. Natarajan, Chem. Commun., 2007, 4471–4473 RSC; (i) W. X. Chen, S. T. Wu, L. S. Long, R. B. Huang and L. S. Zheng, Cryst. Growth Des., 2007, 7, 1171–1175 CrossRef CAS.
- (a) J. K. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729–1733 CrossRef CAS PubMed; (b) B. Hussain, D. Savard, T. J. Burchell, W. Wernsdorfer and M. Murugesu, Chem. Commun., 2009, 9, 1100–1102 RSC.
- X. P. Yang, R. A. Jones and M. J. Wiester, Dalton Trans., 2004, 1787–1788 RSC.
- J. P. Costes and L. Vendier, Eur. J. Inorg. Chem., 2010, 2768–2773 CrossRef CAS.
- (a) J. P. Costes, F. Dahan and F. Nicodeme, Inorg. Chem., 2001, 40, 5285–5287 CrossRef CAS PubMed; (b) A. K. Chaudhari, B. Joarder, E. Rivire, G. Rogez and S. K. Ghosh, Inorg. Chem., 2012, 51, 9159–9161 CrossRef CAS PubMed.
- M. Lalia-Kantouri, C. D. Papadopoulos, A. G. Hatzidimitriou and S. Skoulika, Struct. Chem., 2009, 20, 177–184 CrossRef CAS.
- (a) S. Ghelenji, H. Kargar, Z. Sharafi and R. Kia, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, m1393 CAS; (b) Z. Q. Han, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, m592 CAS; (c) R. Kia, H. Kargar, K. Zare and I. U. Khan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m366 CAS.
- S.-H. Zhang, R.-X. Zhao, G. Li, H. Y. Zhang, Q.-P. Huang and F. P. Liang, J. Solid State Chem., 2014, 220, 206–212 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- (a) H. H. Zou, C. Feng, S. H. Zhang, Y. G. Wang and Y. Xiao, Struct. Chem., 2011, 22, 135–140 CrossRef CAS; (b) P. Even and B. Boitrel, Coord. Chem. Rev., 2006, 250, 519–541 CrossRef CAS; (c) S. Yamada, Coord. Chem. Rev., 1999, 190, 537–555 CrossRef; (d) Y. D. Zhang, S. H. Zhang, C. M. Ge, Y. G. Wang, Y. H. Huang and H. P. Li, Synth. React. Inorg. Met. Org. Chem., 2013, 43(8), 990–994 CrossRef CAS.
- (a) S. T. Meally, C. McDonald, G. Karotsis, G. S. Papaefstathiou, E. K. Brechin, P. W. Dunne, P. McArdle, N. P. Power and L. F. Jones, Dalton Trans., 2010, 39, 4809–4816 RSC; (b) W. L. Leong and J. J. Vittal, New J. Chem., 2010, 34, 2145–2152 RSC; (c) L. Yang, Q. P. Huang, C. L. Zhang, R. X. Zhao and S. H. Zhang, Supramol. Chem., 2014, 26(2), 81–87 CrossRef CAS.
- (a) M. A. Bolcar, S. M. J. Aubin, K. Folting, D. N. Hendrickson and G. Christou, Chem. Commun., 1997, 1485–1486 RSC; (b) R. W. Saalfrank, T. Nakajima, N. Mooren, A. Scheurer, H. Maid, F. Hampel, C. Trieflinger and J. Daub, Eur. J. Inorg. Chem., 2005, 1149–1153 CrossRef CAS; (c) G. L. Abbati, A. Cornia, A. C. Fabretti, A. Caneschi and D. Gatteschi, Inorg. Chem., 1998, 37, 3759–3766 CrossRef CAS PubMed; (d) S. Koizumi, M. Nihei, M. Nakano and H. Oshio, Inorg. Chem., 2005, 44(5), 1208–1210 CrossRef CAS PubMed; (e) S. H. Zhang and C. Feng, J. Mol. Struct., 2010, 977, 62–66 CrossRef CAS; (f) S. Y. Chen, C. C. Beedle, P. R. Gan, G. H. Lee, S. Hill and E. C. Yang, Inorg. Chem., 2012, 51, 4448–4457 CrossRef CAS PubMed; (g) S. K. Langley, N. F. Chilton, B. Moubaraki and K. S. Murray, Dalton Trans., 2012, 41, 9789–9796 RSC; (h) T. Liu, B. W. Wang, Y. H. Chen, Z. M. Wang and S. Gao, Z. Anorg. Allg. Chem., 2008, 634, 778–783 CrossRef CAS; (i) S. Koizumi, M. Nihei, T. Shiga, M. Nakano, H. Nojiri, R. Bircher, O. Waldmann, S. T. Ochsenbein, H. U. Gudel, F. Fernandez-Alonso and H. Oshio, Chem.–Eur. J., 2007, 13, 8445–8453 CrossRef CAS PubMed; (j) R. W. Saalfrank, A. Scheurer, R. Prakash, F. W. Heinemann, T. Nakajima, F. Hampel, R. Leppin, B. Pilawa, H. Rupp and P. Muller, Inorg. Chem., 2007, 46, 1586–1592 CrossRef CAS PubMed.
- M. Tesmer, B. Muller and H. Vahrenkamp, Chem. Commun., 1997, 721–722 RSC.
- (a) H. Oshio, N. Hoshino, T. Ito, M. Nakano, F. Renz and P. Gutlich, Angew. Chem., Int. Ed., 2003, 42, 223–225 CrossRef CAS PubMed; (b) G. Labat, C. Boscovic and H. U. Gudel, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2005, 61, m611–m613 CAS; (c) N. Hoshino, A. M. Ako, A. K. Powell and H. Oshio, Inorg. Chem., 2009, 48, 3396–3407 CrossRef CAS PubMed.
- (a) V. Tudor, G. Marin, F. Lloret, V. C. Kravtsov, Y. A. Simonov, M. Julve and M. Andruh, Inorg. Chim. Acta, 2008, 361, 3446–3452 CrossRef CAS; (b) L. F. Chibotaru, L. Ungur, C. Aronica, H. Elmoll, G. Pilet and D. Luneau, J. Am. Chem. Soc., 2008, 130, 12445–12455 CrossRef CAS PubMed; (c) A. Ferguson, A. Parkin, J. Sanchez-Benitez, K. Kamenev, W. Wernsdorfer and M. Murrie, Chem. Commun., 2007, 3473–3475 RSC; (d) R. Pattacini, P. Teo, J. Zhang, Y. h. Lan, A. K. Powell, J. Nehrkorn, O. Waldmann, T. S. A. Hor and P. Braunstein, Dalton Trans., 2011, 40, 10526–10534 RSC; (e) M. Moragues-Canovas, C. E. Talbot-Eeckelaers, L. Catala, F. Lloret, W. Wernsdorfer, E. K. Brechin and T. Mallah, Inorg. Chem., 2006, 45, 7038–7040 CrossRef CAS PubMed; (f) A. A. Kitos, C. G. Efthymiou, C. Papatriantafyllopoulou, V. Nastopoulos, A. J. Tasiopoulos, M. J. Manos, W. Wernsdorfer, G. Christou and S. P. Perlepes, Polyhedron, 2011, 30, 2987–2996 CrossRef CAS; (g) S. T. Meally, C. McDonald, P. Kealy, S. M. Taylor, E. K. Brechin and L. F. Jones, Dalton Trans., 2012, 41, 5610–5616 RSC; (h) X. T. Wang, B. W. Wang, Z. M. Wang, W. Zhang and S. Gao, Inorg. Chim. Acta, 2008, 361, 3895–3902 CrossRef CAS; (i) W. Wang, H. Hai, S. H. Zhang, L. Yang and C. L. Zhang, J. Cluster Sci., 2014, 25(2), 357–365 CrossRef CAS; (j) Q. P. Huang, G. Li, H. Y. Zhang, S.-H. Zhang and H. P. Li, Z. Anorg. Allg. Chem., 2014, 640(7), 1403–1407 CrossRef CAS.
- (a) Q. Gao, X. Q. Wang, M. T. Conato, T. Makarenko and A. J. Jacobson, Cryst. Growth Des., 2011, 11, 4632–4638 CrossRef CAS; (b) Y. Xiao, S. H. Zhang, G. Z. Li, Y. G. Wang and C. Feng, Inorg. Chim. Acta, 2011, 366, 39–43 CrossRef CAS.
- K. O. Kongshaug and H. Fjellvåg, Solid State Sci., 2003, 5, 303–310 CrossRef CAS.
- P. M. Forster and A. K. Cheetham, Microporous Mesoporous Mater., 2004, 73, 57–64 CrossRef CAS.
- Complex a can be prepared in a similar way than 1 except that Hheb was replaced by Hhmb. Green crystals of a were collected by filtration, washed with methanol and dried. Phase pure crystals of a were obtained by manual separation (yield: 278 mg, ca. 54.71% based on Hhmb ligand). Crystal data for a heterometallic tetranuclear cluster b: C40H40Ni2N10Na2O12, Mr = 1016.22 g mol−1, monoclinic, P21/C, a = 13.405 (1), b = 12.065 (1), c = 14.665 (1) Å, β = 106.73 (1)°, V = 2271.4 (3) Å3, θ = 26.32°, λ = 0.71073Å, T = 293 K, μ(Mo Kα) = 0.920 mm−1. 9947 reflections were collected of which 4001 were unique (Rint = 0.0285). The structure was solved by direct methods and refined by full-matrix least squares of F2, R1 = 0.0436(I > 2σ(I)), wR2 = 0.1501 (all data). Max/min residual electron density 0.464/−0.358 e Å3 and the structure of (b) see Fig. S4.†.
- Complex b can be prepared in a similar way than a except that Ni(ClO4)2·6H2O was replaced by Co(ClO4)2·6H2O. Red crystals of b were collected by filtration, washed with methanol and dried. Phase pure crystals of 2 were obtained by manual separation (yield: 243 mg, ca. 47.80% based on Hhmb ligand). Crystal data for a heterometallic tetranuclear cluster a: C40H40Co2N10Na2O12, Mr = 1016.66 g mol−1, monoclinic, P21/C, a = 13.345 (2), b = 12.088 (2), c = 14.716 (2) Å, β = 106.13 (1)°, V = 2280.6 (5) Å3, θ = 28.99°, λ = 0.71073 Å, T = 293 K, μ(Mo Kα) = 0.818 mm−1. 7824 reflections were collected of which 3907 were unique (Rint = 0.1519). The structure was solved by direct methods and refined by full-matrix least squares of F2, R1 = 0.0939(I > 2σ(I)), wR2 = 0.2689 (all data). Max/min residual electron density 0.838/−0.517 e Å3 and the structure of (a) see Fig. S4.†.
- A. Sieber, C. Boskovic, R. Bircher, O. Waldmann, S. T. Ochsenbein, G. Chaboussant, H. U. Güdel, N. Kirchner, J. V. Slageren, W. Wernsdorfer, A. Neels, H. Stoeckli-Evans, S. Janssen, F. Juranyi and H. Mutka, Inorg. Chem., 2005, 44, 4315–4325 CrossRef CAS PubMed.
- M. A. Halcrow, J. C. Huffman and G. Christou, Angew. Chem., Int. Ed., 1995, 34, 889–891 CrossRef CAS.
- (a) M. A. Halcrow, J. S. Sun, J. C. Huffman and G. Christou, Inorg. Chem., 1995, 34, 4167–4177 CrossRef CAS; (b) B. Cage, F. A. Cotton, N. S. Dalal, E. A. Hillard, B. Rakvin and C. M. Ramsey, J. Am. Chem. Soc., 2003, 125, 5270–5271 CrossRef CAS PubMed; (c) L. Feng, C. C. Beedle, W. Wernsdorfer, C. Koo, M. Nakano, S. Hill and D. N. Hendrickson, Inorg. Chem., 2007, 46, 8126–8128 CrossRef PubMed; (d) M. L. Tong, M. Monfort, J. M. C. Juan, X. M. Chen, X. H. Bu, M. Ohba and S. Kitagawa, Chem. Commun., 2005, 2, 233–235 RSC.
- Y. Song, P. Zhang, X. M. Ren, X. F. Shen, Y. Z. Li and X. Z. You, J. Am. Chem. Soc., 2005, 127, 3708–3709 CrossRef CAS PubMed.
- (a) G. Aromí, H. Stoeckli-Evans, S. J. Teat, J. Cano and J. Ribas, J. Mater. Chem., 2006, 16, 2635–2644 RSC; (b) B. N. Figgis, M. Gerloch, J. Lewis, F. E. Mabbs and G. A. Webb, J. Chem. Soc. A, 1968, 2086–2093 RSC.
- E. K. Brechin, O. Cador, A. Caneschi, C. Cadiou, S. G. Harris, S. Parsons, M. Vonci and R. E. P. Winpenny, Chem. Commun., 2002, 1860–1861 RSC.
- Y. Z. Zhang, W. Wernsdorfer, F. Pan, Z. M. Wang and S. Gao, Chem. Commun., 2006, 31, 3302–3304 RSC.
- (a) M. Murrie, S. J. Teat, H. Stoeckli-Evans and H. U. Güdel, Angew. Chem., Int. Ed., 2003, 42, 4653–4656 CrossRef CAS PubMed; (b) E. C. Yang, D. N. Hendrickson, W. Wernsdorfer, M. Nakano, L. N. Zakharov, R. D. Sommer, A. L. Rheingold, M. Ledezma-Gairaud and G. J. Christou, Appl. Phys., 2002, 91, 7382–7384 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The IR of 1–5. Packing drawing of 3–5. The structures of a and b. The PXRDs of 1–5. The plots of χM−1 vs. T of 1–5. The plots of M–H of 1–5. The plots of χ′′ and χ′ vs. T of 1–5. CCDC 982504–982510. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra09687h |
| This journal is © The Royal Society of Chemistry 2014 |