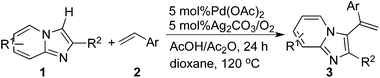
Palladium(II)-catalyzed intermolecular oxidative C-3 alkenylations of imidazo[1,2-a]pyridines by substrate-contolled regioselective C–H functionalization†
Hua Cao*,
Sai Lei,
Jinqiang Liao,
Jianping Huang,
Huifang Qiu,
Qinlin Chen,
Shuxian Qiu and
Yaoyi Chen
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Guangzhou 510006, P. R. China. E-mail: caohua@gdpu.edu.cn; Fax: +86 760 88207939
First published on 30th September 2014
Abstract
An efficient and highly regioselective palladium(II)-catalyzed oxidative C-3 alkenylation of imidazo[1,2-a]pyridines with acrylate, acrylonitrile, or vinylarenes has been developed by using oxygen as an oxidant. Substrates such as acrylate and acrylonitrile tended to form β-product, while vinylarenes tended to form the sole α-products.
The metal-catalyzed Heck reaction which received the Nobel Prize in 2010 has become one of the most widely used methods in chemical synthesis.1 This approach offers the possibility for catalytic transformation of unactivated C–H bonds into diverse functionalities. Currently, direct oxidative alkenylation of heterocycles has already achieved widespread acceptance within the organic synthetic fields, because of its capacity to utilize simpler and cheaper precursors for the preparation of functionalized molecules. Consequently, the discovery of efficient methods for the assembly of carbon–carbon bonds has attracted attention in this field, which avoided synthesis of complex and expensive starting substrates. Since the pioneering Fujiwara and co-workers2 have first described a wealth of palladium- and rhodium-catalyzed oxidative alkenylations. Many elegant direct oxidative alkenylations3 have been achieved without the need for prior halogenation or metallization in this field. Despite of several methods have been reported during the last decades, there is still an intrinsic need for open new routes to synthesize of diverse heterocycles molecules.
Imidazo[1,2-a]pyridines are extremely important chemicals that exhibit a wide range of biological activities4 and are used as antiviral,5 antiulcer,6 antibacterial,7 antifungal,8 antiprotozoal,9 antiherpes,10 anti-inflammatory.11 Since the wide application of imidazo[1,2-a]pyridines in pharmaceutical research and drugs including Alpidem, Zolpidem, Necopidem, Olprinone, Divalpon and Zolimidine are available in the market, the development of efficient methods to synthesize imidazo[1,2-a]pyridines has continuously attracted the attentions of many chemists.12 We have recently developed facile C–H transformation for the preparation of imidazo[1,2-a]pyridine13 and other heterocyclic compounds. In this context, our attention is focused on the development alkenylations of imidazo[1,2-a]pyridine to prepare imidazo[1,2-a]pyridine derivatives (Scheme 1). Moreover, the reaction has proceeded by using molecular oxygen as terminal oxidant, which avoided the environmentally hazardous by-products obtained with other oxidants.14
The initial screening studies have been carried out using 1a and 2a as model substrate to identify and optimize many different combinations of potential catalysts, oxidants, additives, and solvents in order to improve the yields of the reaction. The results are summarized in Table 1. First, treatment of 1a with 2a in the presence of PdCl2, Cu(OAc)2 and AcOH at 120 °C in dioxane, a trace of the desired directly alkenylation product 3a was observed (entry 1). Other Pd-catalyst was also tested (entries 2–5), including Pd(O2CCF3)2, Pd(CH3CN)Cl2, Pd(dba)2, Pd(OAc)2. Interestingly, the use of Pd(OAc)2 afforded the corresponding products 3a in 9% GC yield, while other palladium sources gave poor yields. On changing the oxidants we observed a significant improvement by using Ag2CO3 as oxidant in the reaction and the product 3a was obtained in 32% GC yield (entry 6). Other silver salts, such as AgOAc or AgOTf, were also employed and the desired product was obtained in 21%, 20% yields respectively (Table 1, entries 7 and 8). Other oxidants, such as BQ, DDQ, tBuOOBz, O2 and air were also tested (entries 9–13). The results showed that O2 was a choice for this transformation. To our delight, the good yield was obtained, when the reaction was carried out by using Ag2CO3 (5 mol%) and O2 (with oxygen ballon) as co-oxidant (entry 14) in the presence of Pd(OAc)2. These results encouraged us to adjust additives to improve the yield (entries 15–18). We were pleased to find that the yield of 3a could be observed in 81% by using AcOH and Ac2O as additives (entry 16). Effects of solvents and temperatures were also investigated in the following tests (entries 19–22). Among them, dioxane was demonstrated to be the best choice. Nonetheless, the yield could not be improved with the increasing reaction time to 30 h (entry 23). But decreasing the time to 12 h did affect the reaction efficiency and the low yields of 3a were obtained (entry 24).
| Entry | Catalyst | Oxidant | Additive | Solvent | t (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (2.5 mmol), catalyst (5% mol), oxidant (1.2 mmol), additive (0.5 mmol), solvent (3.0 mL).b GC yields.c 5 mol% Ag2CO3, O2 (500 mL). | ||||||
| 1 | PdCl2 | Cu(OAc)2 | AcOH | Dioxane | 24 | trace |
| 2 | Pd(O2CCF3)2 | Cu(OAc)2 | AcOH | Dioxane | 24 | 5 |
| 3 | Pd(CH3CN)Cl2 | Cu(OAc)2 | AcOH | Dioxane | 24 | trace |
| 4 | Pd(dba)2 | Cu(OAc)2 | AcOH | Dioxane | 24 | trace |
| 5 | Pd(OAc)2 | Cu(OAc)2 | AcOH | Dioxane | 24 | 9 |
| 6 | Pd(OAc)2 | AgOAc | AcOH | Dioxane | 24 | 32 |
| 7 | Pd(OAc)2 | Ag2CO3 | AcOH | Dioxane | 24 | 21 |
| 8 | Pd(OAc)2 | AgOTf | AcOH | Dioxane | 24 | 20 |
| 9 | Pd(OAc)2 | BQ | AcOH | Dioxane | 24 | NR |
| 10 | Pd(OAc)2 | DDQ | AcOH | Dioxane | 24 | NR |
| 11 | Pd(OAc)2 | tBuOOBz | AcOH | Dioxane | 24 | NR |
| 12 | Pd(OAc)2 | O2 (1 atm) | AcOH | Dioxane | 24 | 27 |
| 13 | Pd(OAc)2 | Air (1 atm) | AcOH | Dioxane | 24 | 5< |
| 14c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH | Dioxane | 24 | 69 |
| 15c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | PhCO2H | Dioxane | 24 | 12 |
| 16c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | Dioxane | 24 | 81 |
| 17c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | Py | Dioxane | 24 | 40 |
| 18c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | K2CO3 | Dioxane | 24 | 12 |
| 19c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | Toluene | 24 | 46 |
| 20c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | DMA | 24 | 60 |
| 21c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | DMSO | 24 | 13 |
| 22c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | DMF | 24 | 19 |
| 23c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | Dioxane | 30 | 77 |
| 24c | Pd(OAc)2 | O2 (1 atm)/Ag2CO3 | AcOH/Ac2O | Dioxane | 12 | 62 |
With the optimized conditions in hand, we next investigated the scope of this novel highly regioselective alkenylation of imidazo[1,2-a]pyridines with styrene for synthesis of α-products. And the results are described in Table 2. A variety of imidazo[1,2-a]pyridines with electrondonating methyl groups at the 2-, 6-, 7-, and 8-positions were smoothly alkenylated at the 3-position with styrene in good yields (Table 2). Thus, we turned our attention to examine the scope of vinylarenes. Various substituted styrene was reacted well with 1 and led to the desired α-products 4a–4r in good yields. The presence of electron-withdrawing groups, such as F and Cl, were tolerated in the reaction and afforded the desired products smoothly.
| a Isolated yields. |
|---|
 |
Subsequently, a variety of acrylates were examined. The desired α-product was not formed, while only β-products obtained under the optimized conditions. The results are described in Table 3. A variety of acrylates were examined. It was observed that alteration of an alkoxy part of acrylate did not change the reaction efficiency, and a similar level of product yields was obtained in the alkenylation of butyl, methyl, ethyl, and cyclohexyl acrylate. The scope of various types of imidazo[1,2-a]pyridine substrates was also extensively surveyed. The imidazo[1,2-a]pyridine and its derivatives with electrondonating methyl groups at the 2-, 6-, 7-, and 8-positions were smoothly alkenylated at the 3-position with acrylate in good yields. The substrate ethyl imidazo[1,2-a]pyridine-2-carboxylate with electronwithdrawing CO2Et group at 2-positions also performed very well and afforded the desired product 4j in good yield. Notably, high regioselectivity was observed, when the reactions were carried out by using 2,3-dihydro-imidazo[1,2-a]pyridines with acrylate. All the results indicated that this process is highly regioselective for C-3 alkenylation.
| a Isolated yields. |
|---|
 |
On the basis of the previous report and our results at this stage, we have proposed two plausible pathways for the two oxidative coupling reactions in Scheme 2. Path I:2,15 palladation at C-3 of imidazo[1,2-a]pyridine (electrophilic substitution) was thought to occur intermediate A with the aid of oxidant. Active intermediate A then inserts into the methyl acrylate to give intermediate B, which rapidly decomposes through β-elimination to generate the desired product and Pd catalyst (regular Heck-reaction product); Path II:1b,3l,16 palladium(II) salts tend to react with styrene to give the intermediate C, which then can undergo an intermolecular nucleophilic attack of 1a (usually at the more substituted vinylic carbon) to generate intermediate D. The corresponding product is formed via β-hydride elimination from intermediate D and releases the Pd catalyst.
In summary, we have developed an efficient method for the selective intermolecular alkenylation of substituted imidazo[1,2-a]pyridines with diverse acrylate, acrylonitrile, and styrenes through a palladium-catalyzed C–H functionalization reaction. It represents a novel, regio- and stereoselective oxidative alkenylation process. This transformation using molecular oxygen to avoid excessive silver levels also results in a clean and rather waste-free process.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (21302023) and the Project of Department of Education of Guangdong Province (2013kjcx0111).Notes and references
- (a) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS PubMed; (b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (c) A. d. Meijere and F. E. Meyer, Angew. Chem., Int. Ed., 1994, 34, 2379 Search PubMed.
- (a) I. Moritani and Y. Fujiwara, Tetrahedron Lett., 1967, 8, 1119 CrossRef; (b) Y. Fujiwara, I. Moritani, S. Danno, R. Asano and S. Teranishi, J. Am. Chem. Soc., 1969, 91, 7166 CrossRef CAS.
- (a) S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529 CrossRef CAS; (b) C. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura and Y. Fujiwara, Science, 2000, 287, 1992 CrossRef CAS; (c) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (d) L. Ackermann and J. Pospech, Org. Lett., 2011, 13, 4153 CrossRef CAS PubMed; (e) L. Ackermann, A. V. Lygin and N. Hofmann, Org. Lett., 2011, 13, 3278 CrossRef CAS PubMed; (f) L. Ackermann and S. Fenner, Org. Lett., 2011, 13, 6548 CrossRef CAS PubMed; (g) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC; (h) P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2011, 13, 3075 RSC; (i) Y. H. Zhang, B. F. Shi and J. Q. Yu, J. Am. Chem. Soc., 2009, 131, 5072 CrossRef CAS PubMed; (j) X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (k) H. Q. Do and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 12404 CrossRef CAS PubMed; (l) Y. Yang, K. Cheng and Y. Zhang, Org. Lett., 2009, 11, 5606 CrossRef CAS PubMed; (m) J. J. Li, T. S. Mei and J. Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 6452 CrossRef CAS PubMed; (n) L. Y. Jiao and M. Oestreich, Org. Lett., 2013, 15, 5374 CrossRef CAS PubMed; (o) G. Broggini, V. Barbera, E. M. Beccalli, E. Borsini, S. Gallil, G. Lanza and G. Zecchi, Adv. Synth. Catal., 2012, 354, 159 CrossRef CAS; (p) W. Raufa and J. M. Brown, Chem. Commun., 2013, 49, 8430 RSC; (q) R. C. Jones, M. Gałęzowski and D. F. O'Shea, J. Org. Chem., 2013, 78, 8044 CrossRef CAS PubMed.
- (a) K. S. Gudmundsson and B. A. Johns, Org. Lett., 2003, 5, 1369 CrossRef CAS PubMed; (b) C. Enguehard, F. Fauvelle, J. Debouzy, A. Peinnequin, I. Thery and D. V. A. Gueiffier, Eur. J. Pharm. Sci., 2005, 24, 219 CrossRef PubMed.
- G. Puerstinger, J. Paeshuyse, E. Declercq and J. Neyts, Bioorg. Med. Chem. Lett., 2007, 17, 390 CrossRef CAS PubMed.
- (a) J. J. Kaminsky and A. M. Doweyko, J. Med. Chem., 1997, 40, 427 CrossRef PubMed; (b) Y. Kaesura, S. Nishino, Y. Inoue, M. Tomoi and H. Taksugi, Chem. Pharm. Bull., 1992, 40, 371 CrossRef.
- Y. Rival, G. Grassy and G. Michel, Chem. Pharm. Bull., 1992, 40, 1170 CrossRef CAS.
- Y. Rival, G. Grassy, A. Taudou and R. Ecalle, Eur. J. Med. Chem., 1991, 26, 13 CrossRef CAS.
- M. A. Ismail, R. K. Arafa, T. Wenzler, R. Brun, F. A. Tanious, W. D. Wilson and D. W. Boykin, Bioorg. Med. Chem., 2008, 16, 681 Search PubMed.
- K. S. Gudmundsson and B. A. Johns, Bioorg. Med. Chem. Lett., 2007, 17, 2735 CrossRef CAS PubMed.
- K. C. Rupert, J. R. Henry, J. H. Dodd, S. A. Wadsworth, D. E. Cavender, G. C. Olini, F. Fahmy and J. J. Siekierka, Bioorg. Med. Chem. Lett., 2003, 23, 347 CrossRef.
- (a) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef CAS; (b) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS; (c) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (d) K. S. Masters, T. R. M. Rauws, A. K. Yasav, W. A. Herrebout, B. V. Veken and B. U. W. Mases, Chem.–Eur. J., 2011, 17, 6315 CrossRef CAS PubMed; (e) V. Z. Parchinsky, O. Shuvalova, O. Ushakova, D. V. Kravchenko and M. Krasavin, Tetrahedron Lett., 2006, 47, 947 CrossRef CAS PubMed; (f) F. Chen, M. Lei and L. Hu, Tetrahedron, 2013, 69, 2954 CrossRef CAS PubMed; (g) H. Zhou, W. Wang, O. Khorev, Y. Zhang, Z. Miao, T. Meng and J. Shen, Eur. J. Org. Chem., 2012, 5585 CrossRef CAS; (h) V. Tyagi, S. Khan, V. Bajpai, H. M. Gauniyal, B. Kumar and P. M. S. Chauhan, J. Org. Chem., 2012, 77, 1414 CrossRef CAS PubMed; (i) S. Husinec, R. Markovic, M. Petkovic, V. Nasufovic and V. Savic, Org. Lett., 2011, 13, 2286 CrossRef CAS PubMed; (j) Z. Fei, Y. P. Zhu, M. C. Liu, F. C. Jia and A. X. Wu, Tetrahedron Lett., 2013, 54, 1222 CrossRef CAS PubMed; (k) H. Y. Fu, L. Chen and H. Doucet, J. Org. Chem., 2012, 77, 4473 CrossRef CAS PubMed; (l) H. Yang, L. Yang, Y. Li, F. Zhang, H. Liu and B. Yi, Catal. Commun., 2012, 11 CrossRef PubMed; (m) J. Koubachi, S. El Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, Synthesis, 2008, 2537 CAS; (n) J. Koubachi, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, Synthesis, 2009, 271 CAS.
- (a) H. Cao, H. Y. Zhan, Y. G. Lin, X. L. Lin, Z. D. Du and H. F. Jiang, Org. Lett., 2012, 14, 1688 CrossRef CAS PubMed; (b) H. Cao, Y. G. Lin, H. Y. Zhan, Z. D. Du, X. L. Lin, Q. M. Liang and H. Zhang, RSC Adv., 2012, 2, 5972 RSC; (c) H. Cao, X. H. Liu, L. M. Zhao, J. H. Cen, J. X. Lin, Q. X. Zhu and M. L. Fu, Org. Lett., 2014, 16, 146 CrossRef CAS PubMed.
- (a) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS PubMed; (b) W. Q. Wu and H. F. Jiang, Acc. Chem. Res., 2012, 45, 1736 CrossRef CAS PubMed; (c) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC.
- (a) N. P. Grimster, C. Gauntlett, C. R. A. Godfrey and M. J. Gaunt, Angew. Chem., Int. Ed., 2005, 44, 3125 CrossRef CAS PubMed; (b) E. M. Beck, N. P. Grimster, R. Hatley and M. J. Gaunt, J. Am. Chem. Soc., 2006, 128, 2528 CrossRef CAS PubMed; (c) Y. Yang, H. Gong and C. Kuang, Eur. J. Org. Chem., 2013, 5276 CrossRef CAS; (d) H. Jiang, Z. Feng, A. Wang, X. Liu and Z. Chen, Eur. J. Org. Chem., 2010, 1227 CrossRef.
- (a) W. Cabri and I. Candiani, Acc. Chem. Res., 1995, 28, 2 CrossRef CAS; (b) J. Mo, L. Xu and J. Xiao, J. Am. Chem. Soc., 2005, 127, 751 CrossRef CAS PubMed; (c) H. Schenck, B. Akermark and M. Svensson, J. Am. Chem. Soc., 2003, 125, 3503 CrossRef PubMed; (d) G. Broggini, E. M. Beccalli, A. Fasana and S. Gazzola, Beilstein J. Org. Chem., 2012, 8, 1730 CrossRef CAS PubMed; (e) A. Ashimori, B. Bachand, M. A. Calter, S. P. Govek, L. E. Overman and D. J. Poon, J. Am. Chem. Soc., 1998, 120, 6488 CrossRef CAS; (f) R. J. Deeth, A. Smith and J. M. Brown, J. Am. Chem. Soc., 2004, 126, 7144 CrossRef CAS PubMed; (g) P. Fristrup, S. L. Quement, D. Tanner and P.-O. Norrby, Organometallics, 2004, 23, 6160 CrossRef CAS; (h) C. Bäcktorp and P.-O. Norrby, Dalton Trans., 2011, 40, 11308 RSC; (i) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09669j |
| This journal is © The Royal Society of Chemistry 2014 |