A diketopyrrolopyrrole and benzothiadiazole based small molecule electron acceptor: design, synthesis, characterization and photovoltaic properties†
Abstract
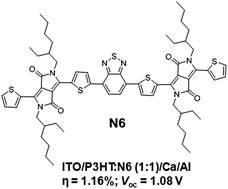
A novel solution-processable electron acceptor based on diketopyrrolopyrrole and benzothiadiazole building blocks was designed and synthesized, which exhibited excellent solubility and thermal stability, and afforded 1.16% power conversion efficiency with high open-circuit voltage (1.08 V) when tested with the classical poly(3-hexylthiophene) electron donor in bulk-heterojunction solar cells. The open-circuit voltage reported here (∼1.1 V) is among the highest values for a single bulk-heterojunction device.

 Please wait while we load your content...
Please wait while we load your content...