pH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery
Abstract
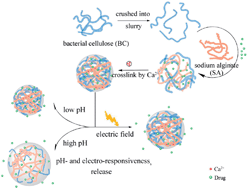
In this work, hybrid hydrogels composed of bacterial cellulose nanofibers (nf-BC) and sodium alginate (SA) were prepared as a dual-stimuli responsive release system. The pH and electric field stimulus-responsive swelling properties and the stimulus-responsive drug release behaviors of the nf-BC/SA hybrid hydrogels using Ibuprofen as a model drug, were investigated in vitro. As the pH changed from 1.5 to 11.8, the swelling ratio increased from less than 8 times compared with its dry weight at acidic conditions to more than 13 times when the pH value was 11.8. When the electric field changed from 0 to 0.5 V, the hybrid hydrogels also showed an increasing swelling ratio from 8 times to 14 times their dry weight. The release of Ibuprofen (IBU) could be controlled by the action of deprotonation or protonation of calcium alginate in the hydrogels under different pH conditions, faster in alkaline conditions and slower in acidic conditions. Furthermore, the drug release from the hybrid hydrogels could be enhanced with an applied electric stimulus. The drug release mechanism under pH and applied electric field could be interpreted by the superposition of both Fickian diffusion and case-II transport based on Peppas' semi-empirical equation. The nf-BC/SA hybrid hydrogels with dual pH- and electro-response were new promising candidates as controlled drug delivery systems.

 Please wait while we load your content...
Please wait while we load your content...