Involvement of process parameters and various modes of application of TiO2 nanoparticles in heterogeneous photocatalysis of pharmaceutical wastes – a short review
Abstract
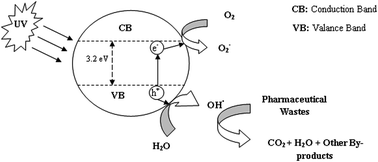
In recent years, the occurrence of persistent organic compounds in industrial as well as municipal effluents is becoming a serious threat to the environment. The pharmaceutical compounds present along with those organics have a detrimental effect on our environment. Among various well established wastewater treatment technologies, advanced oxidation processes (AOPs) using TiO2 nanoparticles have shown promising results against various organic wastewater pollutants. This study represents an in-depth review of applications of TiO2 in the treatment of various pharmaceutical wastes and the effects of associated process controlling parameters. It also highlights aspects of different application techniques of TiO2 nanoparticles and the involvement of reaction kinetics in photocatalytic degradation.

 Please wait while we load your content...
Please wait while we load your content...