One-pot synthesis of 1,2-diphenyl-1H-benzo[d]imidazole derivatives by a Pd-catalyzed N-arylation and Cu-catalyzed C–H functionalization/C–N bond formation process†
Manman Sun,
Chenhui Chen and
Weiliang Bao*
Department of Chemistry, Zhejiang University (Xixi Campus), Hangzhou 310028, People's Republic of China. E-mail: wlbao@css.zju.edu.cn; Fax: +86-571-88273814; Tel: +86-571-88273814
First published on 19th September 2014
Abstract
A one-pot synthesis of 1,2-diphenyl-1H-benzo[d]imidazole derivatives starting from N-phenylbenzimidamides and iodobenzenes or bromobenzenes has been introduced. The process consisted of a Pd-catalyzed N-arylation and a Cu-catalyzed C–H functionalization/C–N bond formation.
Metal-catalyzed C–H functionalization, because of its atom economy and environmental benefits, has been intensively investigated in the past decade.1 C–H functionalization/C–N bond formation has been a promising strategy to prepare N-heterocycles from simple precursors,2 which exist as fundamental motifs in the scaffold of more than half of the biological molecules and pharmaceutical products. Recently, more concise cascade, tandem, and domino reactions involving C–H functionalization/C–N bond formation attract chemists of increasing interest,3 because these processes avoid the tedious step by step separations and purifications of intermediates and reduce the amount of pollutant waste. Amidine is a type of easily obtainable compound and often used as synthetic precursor. Tandem reactions starting from amidines have been reported by many groups to synthesize diverse benzimidazoles,12–15 quinazolinones,4 quinazoline,5 pyrimidines6 and imidazoles.7 On the other hand, a broad variety of Pd-catalyzed coupling reactions of N–H nucleophiles with C–X (X = Br, I) bonds,8,14b and Cu-catalyzed oxidative cross-dehydrogenative-couplings (CDC) of C–H bonds with N–H bonds9,12a have also been reported. Obviously, a process of Pd-catalyzed N-arylation and Cu-catalyzed C–H functionalization/C–N bond formation in one-pot starting from amidines is more concise.
Benzimidazole motif is often found in commercial medicine.10 Therefore, it is not surprising that the efficient synthesis of benzimidazoles is always in high demand for organic and medicinal chemistry.11 In the past decade, some interesting literatures using amidines to synthesize benzimidazoles have been reported. Buchwald, Shi and Zhu groups reported the synthesis of 1-H-2-substituted benzimidazoles through intramolecular-cyclization of N-phenylbenzimidamides.12 Brain, Deng and Punniyamurthy groups reported the synthesis of 1,2-disubstituted benzimidazoles through intramolecular aryl-amination of N′-(2-halophenyl)-N-phenylbenzimidamides.13 Deng and You groups reported the synthesis of 1,2-disubstituted benzimidazoles through intermolecular tandem amination, starting from 1,2-dihaloarenes and 1,2-disubstituted amidines.14 Zhu group reported an one-pot synthesis of 1,2-disubstituted benzimidazoles by a Chan–Lam–Evans N-arylation and C–H activation/C–N bond forming process, starting from organoboronic acids and 1,2-disubstituted amidines.15 In this paper, we report a more efficient and economic one-pot synthesis of 1,2-diphenyl-1H-benzo[d]imidazole derivatives from N-phenylbenzimidamides and iodo- or bromobenzenes. The 1,2-diphenyl-1H-benzo[d]imidazole derivatives have been used as cyclometalated ligands or important components to prepare iridium(III) or platinum(II) complexes that exhibit improved luminescence properties and stability for applications.16
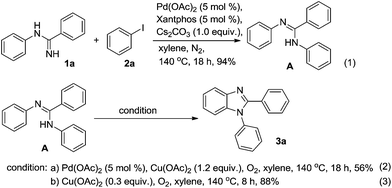
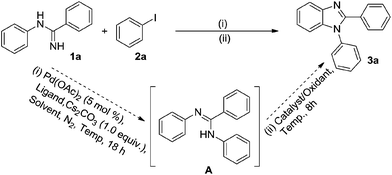
Firstly, we studied the reaction stepwise (Scheme 1). Initially, equivalent N-phenylbenzimidamide 1a and iodobenzene 2a were examined to form the coupling product A. When Pd(OAc)2 (5 mol%), xantphos (5 mol%) and Cs2CO3 (1.0 equiv.) were added, and the mixture was reacted in xylene under nitrogen atmosphere at 140 °C for 18 h, nearly equivalent product A was isolated (Scheme 1(1), 94% yield). Then the A was further examined to synthesize the final cyclized product 3a. When Pd(OAc)2 (5 mol%) was catalyst and Cu(OAc)2 (1.2 equiv.)/O2 was oxidant, the desired product 3a was obtained in 56% yield after reacted in xylene at 140 °C for 18 h (Scheme 1(2)). Excitingly, when no Pd(OAc)2 was added, Cu(OAc)2 was reduced to 0.3 equiv. and O2 was the final oxidant, the desired product 3a was obtained in satisfactory yield of 88% after 6 h in xylene at 140 °C (Scheme 1(3)). Therefore, we obtained the best reaction condition for stepwise process. Based on these conditions, the one-pot reaction condition was optimized as follows (Table 1).
| Entry | (i) | (ii) | Yieldb (%) | ||
|---|---|---|---|---|---|
| Ligand (5 mol%) | Temp (°C) | Catalyst (equiv.)/oxidant | Temp (°C) | ||
| a Reaction conditions: (i) 1a (0.3 mmol), 2a (0.3 mmol), Pd(OAc)2 (5 mol%, 0.015 mmol), ligand (5 mol%, 0.015 mmol), Cs2CO3 (0.3 mmol) in solvent under N2 for 18 h, then (ii) catalyst/oxidant was added for 8 h.b Isolated yield of 3a.c Xantphos = 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene.d 2a was 1.5 equiv. and the reaction time of step (ii) was 30 hourse dppp = 1,3-bis(diphenyphosphino)propane.f dppf = 1,1′-bis(diphenyphosphino)ferrocene.g NMP was solvent.h DMSO was solvent. | |||||
| 1 | Xantphosc | 140 | Cu(OAc)2 (0.3)/O2 | 140 | 82 |
| 2 | Xantphos | 140 | Cu(OAc)2 (1.2)/air | 140 | 54 |
| 3 | Xantphos | 140 | O2 | 140 | 10 |
| 4 | Xantphos | 140 | Cu(OTf)2 (0.3)/O2 | 140 | 50 |
| 5 | Xantphos | 140 | FeCl3 (0.3)/O2 | 140 | 5 |
| 6 | Xantphos | 140 | FeBr3 (0.3)/O2 | 140 | 10 |
| 7 | Xantphos | 120 | Cu(OAc)2 (0.3)/O2 | 120 | 31 |
| 8 | Xantphos | 120 | Cu(OAc)2 (0.3)/O2 | 140 | 55 |
| 9d | Xantphos | 140 | Cu(OAc)2 (0.3)/O2 | 140 | 67 |
| 10 | PPh3 | 140 | Cu(OAc)2 (0.3)/O2 | 140 | Trace |
| 11 | dpppe | 140 | Cu(OAc)2 (0.3)/O2 | 140 | Trace |
| 12 | dppff | 140 | Cu(OAc)2 (0.3)/O2 | 140 | Trace |
| 13g | Xantphos | 140 | Cu(OAc)2 (0.3)/O2 | 140 | 31 |
| 14h | Xantphos | 140 | Cu(OAc)2 (0.3)/O2 | 140 | Trace |
Equivalent N-phenylbenzimidamide 1a and iodobenzene 2a, Pd(OAc)2 (5 mol%), xantphos (5 mol%) and Cs2CO3 (1.0 equiv.) were mixed in an over-dried Schlenk tube, and the mixture was reacted in xylene under nitrogen atmosphere at 140 °C for 18 h, then Cu(OAc)2 (0.3 equiv.)/O2 was added. The desired product 3a was obtained in exciting yield of 82% (Table 1, entry 1). To increase the yield of 3a, we examined the condition of step (ii) firstly. When 1.2 equiv. Cu(OAc)2 was added as oxidant, and the reaction of step (ii) was conducted in air, the yield of 3a was 54% (Table 1, entry 2). When only O2 atmosphere was applied to the reaction step (ii), few of 3a was obtained (Table 1, entry 3). So as a catalyst, Cu2+ may be necessary, and O2 may be a final oxidant. Unfortunately, after testing other copper sources and iron sources, no better system was obtained than Cu(OAc)2/O2 (Table 1, entries 4–6). Reducing the temperature of step (i) or step (ii) caused diminution in the yield (Table 1, entries 7 and 8). When the proportion of 2a was raised to 1.5 equiv., the reaction time of step (ii) should be extended to 30 hours, and the yield of 3a was decreased to 67% (Table 1, entry 9). Then three phosphate ligands and two solvents were screened, xantphos and xylene were found to be superior (Table 1, entries 10–14). Eventually, the conditions described in entry 1 were selected as the optimal conditions.
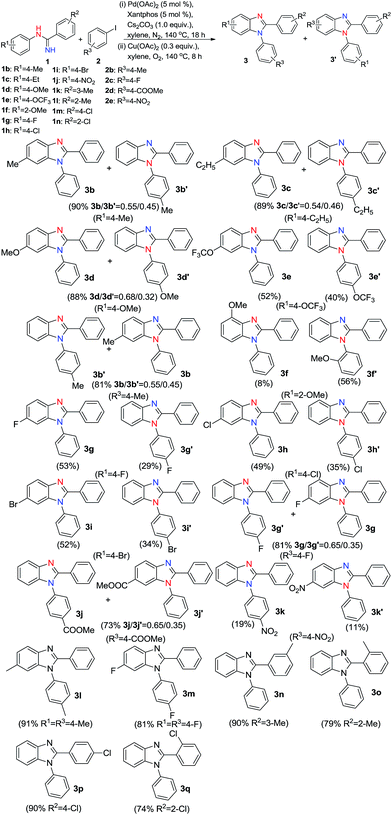
With the optimized reaction conditions in hand, a variety of substituted N-phenylbenzimidamides and iodobenzenes were tested to explore the scope and the generality of the one-pot reaction (Scheme 2). From the scheme, it could be seen that if substituted groups R1 and R3 were different, there would be two benzimidazole isomers produced. When R1 or R3 was p-electron-donating group, such as –CH3, –C2H5, –OMe or –OCF3, excellent yield was obtained, and the main isomer was 6-substituted-1,2-diphenyl-1H-benzo[d]imidazole 3b, 3c, 3d or 3e. When R1 or R3 was moderate p-electron-withdrawing group, such as –F, –Cl or –Br, the yield was a little less than the yield of electron-donating substrate. However, the main isomer was also 6-substituted-1,2-diphenyl-1H-benzo[d]imidazole 3g, 3h or 3i. Maybe there were two tautomeric isomers in intermediate A. When intermediate A underwent the cyclization reaction in the second step, two factors influenced it. In p-electron-donating substituted intermediate A, electron-rich phenyl ring was easier to cyclize with nitrogen.17 In p-halogen substituted intermediate A, the halogens may stabilize the positive metallacycle IV, just like in aromatic electronic substitutions. When R3 was –COOMe, the main isomer was methyl 4-(2-phenyl-1H-benzo[d]imidazol-1-yl)benzoate 3j with moderate yield, cyclization took place on electron-rich phenyl ring. When R1 was –NO2, no desired product was obtained. 4-Nitro-N-phenylaniline was formed instead of the intermediate A. When R3 was –NO2, the final product 3k could be obtained, but the yield was very low. In this instance, the intermediate A could be formed, but a lot of 4-nitro-N-phenylaniline was also formed, so the yield was low. The mainly produced isomer was also 1-(4-nitrophenyl)-2-phenyl-1H-benzo[d]imidazole 3k, cyclization also took place on electron-rich phenyl ring. When R1 and R3 were the same, no isomer was generated. However, comparing 3n with 3o, and 3p with 3q, it could be seen that the yields of o-substituted products were lower than p-substituted and m-substituted products. It directly showed the steric hindrance. This steric effect could also be seen in the reaction of N-(2-methoxyphenyl)benzimidamide 1f with 2a. In this reaction, not only the yield was obviously decreased, but also main isomer was changed to 1-(2-methoxyphenyl)-2-phenyl-1H-benzo[d]imidazole 3f′.
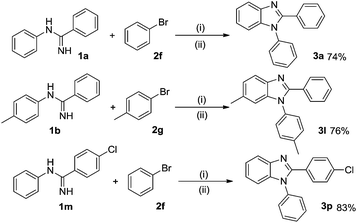
Taking economic factor into account, bromobenzene 2f and 1-bromo-4-methylbenzene 2g were reacted with 1a, 1b or 1m under the optimized reaction conditions in Scheme 3. To our delight, these reactions could also be well conducted with acceptable yields, though a little less than the corresponding yields with iodobenzenes.
According to all the above results, a plausible mechanism was proposed as shown in Scheme 4. Firstly the oxidative addition of iodobenzene 2a to Pd0 generated aryl palladium iodine complex I. Then the amidine 1g was coordinated to I, and then an amido complex II was formed. Subsequent reductive elimination of II released the intermediate aryl amidine A and regenerated the Pd0 catalyst. A existed in a mixture of two isomers. The mechanical route of one isomer was described as follows. Under the action of Cu(OAc)2, a Cu–N adduct III was formed. Then the N-phenyl ring attacked the copper center in III to give a metallacycle IV. Subsequent re-aromatization and elimination processes produced the major product 3g. Copper was released in the Cu(I) state and oxidized by O2 to Cu(II).
Conclusions
In summary, we have introduced a method that Pd-catalyzed N-arylation and Cu-catalyzed C–H functionalization/C–N bond formation in one-pot. This method provides an efficient and concise approach to 1,2-diphenyl-1H-benzo[d]imidazole derivatives in moderate to excellent yields, starting from N-phenylbenzimidamides and iodobenzenes or bromobenzenes.Acknowledgements
This work is financially supported by the Natural Science Foundation of China (no. 21072168).Notes and references
- For recent reviews, see: (a) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (b) S. A. Girard, T. Knauber and C. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed.
- (a) T. Zhang and W. Bao, J. Org. Chem., 2013, 78, 1317 CrossRef CAS PubMed; (b) J. Guan, G. Wu and F. Han, Chem.–Eur. J., 2014, 20, 3301 CrossRef CAS PubMed; (c) J. Hu, H. Xu, P. Nie, X. Xie, Z. Nie and Y. Rao, Chem.–Eur. J., 2014, 20, 3932 CrossRef CAS PubMed; (d) W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS PubMed; (e) M. Wasa and J. Yu, J. Am. Chem. Soc., 2008, 130, 14058 CrossRef CAS PubMed; (f) J. Hu, S. Chen, Y. Sun, J. Yang and Y. Rao, Org. Lett., 2012, 14, 5030 CrossRef CAS PubMed; (g) J. Maes, T. R. M. Rauws and B. U. W. Maes, Chem.–Eur. J., 2013, 19, 9137 CrossRef CAS PubMed.
- (a) G. Wang, T. Yuan and D. Li, Angew. Chem., Int. Ed., 2011, 50, 1380 CrossRef CAS PubMed; (b) J. Karthikeyan and C. Cheng, Angew. Chem., Int. Ed., 2011, 50, 9880 CrossRef CAS PubMed; (c) C. S. Yi, S. Y. Yun and I. A. Guzei, J. Am. Chem. Soc., 2005, 127, 5782 CrossRef CAS PubMed; (d) C. S. Yi and S. Y. Yun, J. Am. Chem. Soc., 2005, 127, 17000 CrossRef CAS PubMed; (e) L. Zeng, W. Yi and C. Cai, Eur. J. Org. Chem., 2012, 559 CrossRef CAS; (f) M. Selvaraju and C. Sun, Adv. Synth. Catal., 2014, 356, 1329 CrossRef CAS; (g) M. Zhao, Z. Ren, Y. Wang and Z. Guan, Chem.–Eur. J., 2014, 20, 1839 CrossRef CAS PubMed.
- X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348 CrossRef CAS PubMed.
- (a) Y. Lv, Y. Li, T. Xiong, W. Pu, H. Zhang, K. Sun, Q. Liu and Q. Zhang, Chem. Commun., 2013, 49, 6439 RSC; (b) Y. Wang, H. Wang, J. Peng and Q. Zhu, Org. Lett., 2011, 13, 4604 CrossRef CAS PubMed.
- J. A. McCauley, C. R. Theberge and N. J. Liverton, Org. Lett., 2000, 2, 3389 CrossRef CAS PubMed.
- (a) J. Li and L. Neuville, Org. Lett., 2013, 15, 1752 CrossRef CAS PubMed; (b) X. Liu, D. Wang, Y. Chen, D. Tang and B. Chen, Adv. Synth. Catal., 2013, 355, 2798 CrossRef CAS.
- (a) Z. Wang, R. T. Skerlj and G. J. Bridger, Tetrahedron Lett., 1999, 40, 3543 CrossRef CAS; (b) B. P. Fors, D. A. Watson, M. R. Biscoe and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 13552 CrossRef CAS PubMed; (c) K. W. Anderson, R. E. Tundel, T. Ikawa, R. A. Altman and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 6523 CrossRef CAS PubMed; (d) J. Barluenga, M. A. Fernández, F. Aznar and C. Valdés, Chem. Commun., 2002, 2362 RSC; (e) Q. Shen, T. Ogata and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 6586 CrossRef CAS PubMed.
- (a) H. He, Z. Wang and W. Bao, Adv. Synth. Catal., 2010, 352, 2905 CrossRef CAS; (b) X. Li, L. He, H. Chen, W. Wu and H. Jiang, J. Org. Chem., 2013, 78, 3636 CrossRef CAS PubMed; (c) M. M. Guru, M. A. Ali and T. Punniyamurthy, Org. Lett., 2011, 13, 1194 CrossRef CAS PubMed; (d) J. Zeng, Y. J. Tan, M. L. Leow and X. Liu, Org. Lett., 2012, 14, 4386 CrossRef CAS PubMed; (e) R. Berrino, S. Cacchi, G. Fabrizi and A. Goggiamani, J. Org. Chem., 2012, 77, 2537 CrossRef CAS PubMed; (f) P. Huang, K. Parthasarathy and C. Cheng, Chem.–Eur. J., 2013, 19, 460 CrossRef CAS PubMed.
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed.
- (a) A. Beu-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1998, 39, 4481 CrossRef; (b) B. Zou, Q. Yuan and D. Ma, Angew. Chem., Int. Ed., 2007, 46, 2598 CrossRef CAS PubMed; (c) N. Zheng, K. W. Anderson, X. Huang, H. N. Nguyen and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 7509 CrossRef CAS PubMed; (d) D. N. Kommi, P. S. Jadhavar, D. Kumar and A. K. Chakraborti, Green Chem., 2013, 15, 798 RSC.
- (a) G. Brasche and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 1932 CrossRef CAS PubMed; (b) Q. Xiao, W. Wang, G. Liu, F. Meng, J. Chen, Z. Yang and Z. Shi, Chem.–Eur. J., 2009, 15, 7292 CrossRef CAS PubMed; (c) J. Huang, Y. He, Y. Wang and Q. Zhu, Chem.–Eur. J., 2012, 18, 13964 CrossRef CAS PubMed; (d) Y. He, J. Huang, D. Liang, L. Liu and Q. Zhu, Chem. Commun., 2013, 49, 7352 RSC.
- (a) C. T. Brain and S. A. Brunton, Tetrahedron Lett., 2002, 43, 1893 CrossRef CAS; (b) C. T. Brain and J. T. Steer, J. Org. Chem., 2003, 68, 6814 CrossRef CAS PubMed; (c) X. Deng, H. McAllister and N. S. Mani, J. Org. Chem., 2009, 74, 5742 CrossRef CAS PubMed; (d) P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719 CrossRef CAS PubMed; (e) P. Saha, M. A. Ali, P. Ghosh and T. Punniyamurthy, Org. Biomol. Chem., 2010, 8, 5692 RSC.
- (a) X. Deng and N. S. Mani, Eur. J. Org. Chem., 2010, 680 CrossRef CAS; (b) D. Zhao, J. Hu, N. Wu, X. Huang, X. Qin, J. Lan and J. You, Org. Lett., 2011, 13, 6516 CrossRef CAS PubMed.
- J. Li, S. Benard, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 5980 CrossRef CAS PubMed.
- (a) H. Cao, H. Sun, Y. Yin, X. Wen, G. Shan, Z. Su, R. Zhong, W. Xie, P. Li and D. Zhu, J. Mater. Chem. C, 2014, 2, 2150 RSC; (b) H. Li, J. Ding, Z. Xie, Y. Cheng and L. Wang, J. Organomet. Chem., 2009, 694, 2777 CrossRef CAS PubMed; (c) H. Cao, G. Shan, Y. Yin, H. Sun, Y. Wu, W. Xie and Z. Su, Dyes Pigm., 2015, 112, 8 CrossRef CAS PubMed; (d) X. Lei, J. Yu, J. Zhao and Y. Jiang, Phys. B, 2011, 406, 4249 CrossRef CAS PubMed; (e) J. Ding, B. Wang, Z. Yue, B. Yao, Z. Xie, Y. Cheng, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2009, 48, 6664 CrossRef CAS PubMed.
- J. Zhu, H. Xie, Z. Chen, S. Li and Y. Wu, Synlett, 2009, 20, 3299 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra and characterizations for all new compounds. See DOI: 10.1039/c4ra09542a |
| This journal is © The Royal Society of Chemistry 2014 |