Fabrication, gradient extraction and surface polarity-dependent photoluminescence of cow milk-derived carbon dots†
Abstract
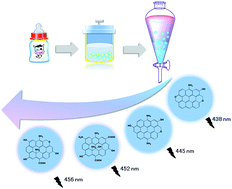
Cow milk-derived carbon dots (CMCD) were separated using a simple and cheap “gradient extraction” method, which was applied for the first time in nanomaterials' separation. The surface polarity of the extracted CMCD correlates well with the polarity of the extraction solvent. Interestingly, the surface polarity also affects the photoluminescence (PL) of CMCD: a red-shift of PL was observed as the surface polarity increased, which was attributed to the increasing amount of polar functional groups on the surface as auxochromes which are bound to graphitic sp2 clusters and reduce their energy gaps. Furthermore, as the surface polarity of CMCD increases, their PL exhibits longer lifetimes and a stronger excitation-dependency, which are attributed to the more efficient “internal” energy transfer from the auxochrome-poor sp2 clusters to the auxochrome-rich sp2 clusters of the CMCD.

 Please wait while we load your content...
Please wait while we load your content...