An all-carbon counter electrode for highly efficient hole-conductor-free organo-metal perovskite solar cells†
Abstract
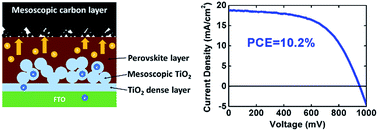
An all-carbon counter electrode has been fabricated for hole-conductor-free organo-metal perovskite heterojunction thin-film solar cells by a simple and low-temperature process. The counter electrode consisted of two parts: a mesoscopic carbon layer for good contact with the perovskite layer, and a piece of industrial flexible graphite sheet as the conducting electrode. Several types of carbon materials were employed in the counter electrodes and tested. From an electrochemical impedance study, it is found that the contact between the counter electrode and perovskite layer has a significant influence on the charge transport properties of the cells. A power conversion efficiency up to 10.2% has been achieved by hole-conductor-free mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with the counter electrode containing a composition of graphite and carbon black, which inspires a new promising route towards low-cost and large-scale commercialization of perovskite solar cells.

 Please wait while we load your content...
Please wait while we load your content...