Studies on FMCM-41 reinforced cyanate ester nanocomposites for low k applications†
Abstract
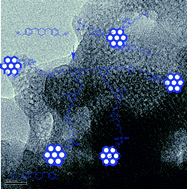
The continual development of microelectronics needs insulation materials with lower dielectric constant (low k). To accomplish this, a new type of cyclohexyl branched aliphatic chain bridged cyanate ester has been developed by the synthesis of chalcone from 4-hydroxybenzaldehyde and cyclohexanone, and followed by reduction. Cyanate ester nanocomposites have been developed by polymerizing cyanate ester monomer reinforced with varying weight percentages of glycidyl silane functionalized mesoporous MCM-41 (FMCM-41). Subsequently, the monomer and polymer composites were characterized by spectral analysis using FTIR (Fourier transform infrared), 1H and 13C NMR (nuclear magnetic resonance) spectroscopy. The thermal properties were analyzed by TGA (thermogravimetric analysis) and DSC (differential scanning calorimetry), and morphological studies were carried out by SEM (scanning electron microscopy) and HRTEM (high resolution transmission electron microscopy). The HRTEM images clearly indicate the existence of pores, which were responsible for the reduction of the dielectric constant. The dielectric properties were measured by broadband dielectric spectrometer. From the dielectric studies it was inferred that 10 wt% of MCM-41 reinforced BCC polymer composites exhibits the lowest value of dielectric constant of 1.98 at 1 MHz.

 Please wait while we load your content...
Please wait while we load your content...