Control of cholesterol homeostasis by entero-hepatic bile transport – the role of feedback mechanisms†
Abstract
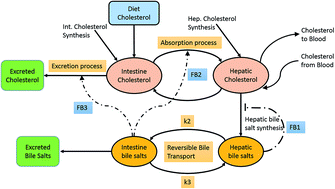
Cholesterol homeostasis is achieved through a tight regulation between synthesis, dietary absorption, utilization of bile salts, and excretion. These processes are regulated through three known feedback mechanisms, namely auto-negative regulation of hepatic bile salt synthesis, and positive regulation of intestinal bile salts on cholesterol absorption and excretion. A model for entero-hepatic cholesterol metabolism in conjunction with dietary inputs for cholesterol was used to obtain insights into the role of the feedback. The analysis demonstrated the significance of the negative feedback in maintaining physiological levels of cholesterol. Furthermore, the positive feedback by the intestinal bile salts on the cholesterol absorption/excretion processes plays an important role in plasma cholesterol homeostasis. Under familial hypercholesterolemia (FH), perturbations in the hepato-intestinal reversible bile transport revealed that an increase in the transport of bile salts from the intestine to the liver decreased the hepatic cholesterol absorption rate. Under such a condition, a twofold decrease in intestinal bile salt transport resulted in an approximately 20% reduction in the plasma cholesterol level, thereby restoring it to normalcy. This suggests that the bile transport control strategy presents an alternative therapeutic method that can effectively reduce cholesterol absorption to attain cholesterol homeostasis.

 Please wait while we load your content...
Please wait while we load your content...