Facile synthesis of azaarene-2-substituted chromanone derivatives via tandem sp3 C–H functionalization/decarboxylation of azaarenes with 4-oxo-4H-chromene-3-carboxylic acid†
Zhuzhou Shao,
Liang Wang,
Lubin Xu,
Huaili Zhao and
Jian Xiao*
College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao, 266109, China. E-mail: chemjianxiao@gmail.com
First published on 3rd October 2014
Abstract
A facile catalyst-free tandem sp3 C–H functionalization/decarboxylation of 4-oxo-4H-chromene-3-carboxylic acid with 2-alkylazaarenes was developed, which can efficiently construct azaarene 2-substituted chromanones in moderate to good yields. This method proves to be efficient and innovative and the biologically significant azaarene 2-substituted chromanones can be accessed in a single step through this methodology.
Over the past years, the concept of a privileged skeleton has arisen and played an important role in drug discovery and development. There is no dispute that chromanone is such a scaffold which represents an important class of naturally occurring structures, characterized by their abilities to interact with a number of different receptors in the body,1 e.g. taxifolin (antibacterial),1g 12-oxocalanolide A (anti-HIV-1),2 aposphaerin A (antibacterial),1i and gonytolide A (innate immune promoter) (Fig. 1).1f,1h,3 Due to their attractive pharmacological and biological properties, great efforts have been devoted to the development of efficient synthetic approaches to access this class of privileged compounds during the past decades.4
The intramolecular Stetter reaction has been demonstrated to be a powerful strategy for the synthesis of 3-substituted chromanones,4d–h while 2-substituted chromanones can be facilely accessed via 1,4-conjugate addition of various nucleophiles to chromones.1d,4c,5 This structure can also be readily furnished via alkyltin-free radical methodology6 or intramolecular oxa-Michael cyclization of substituted 2-hydroxy-chromones prepared in situ1c,7 or beforehand.3,8 Remarkably, photo-fries rearrangement has also been exploited to prepare chromanones from readily available (hetero)aryl 3-methyl-2-butenoate esters.9 Fused or spiro chromanones can also be facilely constructed through the direct oxidative coupling,10 1,3-dipolar cycloaddition,11 Diels–Alder reaction,12 etc.
The azaarene unit is an extremely important pharmacophore which exists in many natural and synthetic bioactive compounds.13 Owing to the high significance of azaarenes and chromanones, it is of vital importance to modify and functionalize these two class of compounds from the viewpoints of both organic and medicinal chemists. However, to the best of our knowledge, no direct method is available to prepare this novel and biologically important azaarene-2-substituted chromanones despite their potential to be new biologically important compounds and new pharmaceuticals.
Substituted azaarene derivatives can be readily accessed via activation of 2-alkylazaarenes catalyzed by a variety of transition metals, such as scandium,14 copper,15 palladium,16 iron17 and ytterbium.18 Recently, significant progresses have been achieved for direct sp3 C–H functionalization of 2-alkylazaarenes without resorting to the toxic transition metals.19,20a,b In 2012, we reported the first metal-free and Brønsted acid catalyzed sp3 C–H functionalization of 2-alkylazaarenes with isatins (Scheme 1, 1a).20a On the basis of that work, a catalyst-free direct sp3 C–H functionalization of 2-alkylazaarenes was further developed in our group via a tandem Michael addition/decarboxylation of 2-alkylazaarenes and (thio)coumarin-3-carboxylic acid (Scheme 1, 1b).20b As a continuation of development of efficient and green methodology to construct biologically and pharmaceutically important molecules,20 herein we report a catalyst-free tandem sp3 C–H activation/decarboxylation of 2-alkylazaarenes with 4-oxo-4H-chromene-3-carboxylic acids which furnished the azaarene 2-substituted chromanone derivatives in one step (Scheme 1c).
From our previous work,20b we have learned that carboxylic group introduced into electron-deficient olefins can serve as Brønsted acid for activation of 2-alkylazaarenes, triggering the reaction in the absence of catalyst. As a further research of this chemistry, 4-oxo-4H-chromene-3-carboxylic acid was selected as the substrate to undertake this tandem reaction, aiming to construct azaarene 2-substituted chromanone derivatives in one step. To validate our proposal, the initial investigation was conducted via the reaction of 4-oxo-4H-chromene-3-carboxylic acid 1 and 2,6-lutidine 2a under catalyst-free condition in dioxane at 140 °C (Table 1, entry 5). Gratifyingly, the reaction proceeded successfully and the coupled product 3a was obtained in 67% yield. Notably, only one methyl of 2,6-lutidine was functionalized, which might be rationalized that the second functionalization of 3a was blocked because of the increased steric hindrance in 3a. The employment of other solvents only led to inferior yields (Table 1, entries 1–4). Afterwards, a variety of Lewis acids and Brønsted acids (10 mol%) were examined at 140 °C for comparison with the catalyst-free condition. It was intriguing to find that almost all the tested Lewis acids and Brønsted acids, such as Sc(OTf)3, La(OTf)3, AgOTf, TfOH, HOAc (Table 1, entries 6–9 and 11), gave inferior yields except TFA (Table 1, entry 10), which were in sharp contrast with the effective sp3 C–H activation reaction catalyzed by Lewis and Brønsted acids.14–19,20a Thus our hypothesis was eventually corroborated.
| Entry | Solvent | Catalyst | Yieldb (%) |
|---|---|---|---|
| a Reaction were conducted with 1 (0.3 mmol), 2a (0.75 mmol) in 2 mL solvent at 140 °C for 48 h.b The yields indicated are the isolated yield by column chromatography. | |||
| 1 | DMF | — | 38 |
| 2 | THF | — | 39 |
| 3 | Toluene | — | 35 |
| 4 | DCE | — | 26 |
| 5 | 1,4-Dioxane | — | 67 |
| 6 | 1,4-Dioxane | Sc(OTf)3 | Trace |
| 7 | 1,4-Dioxane | La(OTf)3 | 16 |
| 8 | 1,4-Dioxane | AgOTf | 65 |
| 9 | 1,4-Dioxane | TfOH | 23 |
| 10 | 1,4-Dioxane | TFA | 70 |
| 11 | 1,4-Dioxane | AcOH | Trace |
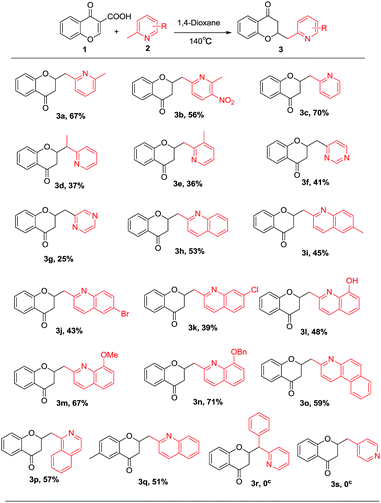
Subsequently the substrate scope was investigated and the results are shown in Scheme 2. A series of electronically and sterically diverse 2-alkyl substituted azaarenes were tolerated under the optimized condition. The position of alkyl group was critical to the success of this tandem process. For example, the methyl of 2-methyl pyridine and 3-nitro-2,6-lutidine could be smoothly functionalized (3b, 3c); whereas the 3-methyl or 4-methyl on pyridine substrates could not be tolerated in this reaction (3e and 3r). The steric hindrance dramatically influenced the yields of the products for example yields of 3d, 3e were decreased when compared with that of 3c and 3q. Notably, free OH group can be tolerated in this reaction (3l). The electron-donating groups such as OMe and OBn were beneficial to this reaction (3m, 3n). However, when nitro group was introduced to para-position of 2,6-lutidine, the yield was decreased to 56% (3b), which might be ascribed to electron-deficiency caused by nitro group. Additionally, 2-methylquinoline (3h), 1-methylisoquinoline (3p), 2,6-dimethylquinoline (3i) and 6-bromo-2-methylquinoline (3j) were also well-tolerated to produce the analogous coupled products in moderate to good yields. Moreover, the methyl of 4-methylpyrimidine (3f) and 2-methylpyrazine (3g) could also be functionalized. As to 3j and 3k, the bromo- and chloro-substituents are versatile synthetic handles which can be further elaborated in miscellaneous means, e.g. transition metal catalyzed couple reaction and lithium–halogen exchange reaction. Other 4-oxo-4H-chromene-3-carboxylic acids such as 6-methyl-4-oxo-4H-chromene-3-carboxylic acid can also work for this reaction to give the desired product 3q in 51% yield.
To explicate the reaction mechanism, 3-acetyl-4H-chromen-4-one 4 and 4-oxo-4H-chromene-3-carbaldehyde 5 were prepared and subjected to the optimized condition (Scheme 3). Despite the fact that the acetyl and formyl group are electron-withdrawing groups which can make the alkene more electrophilic, both of them remained intact under the standard reaction condition. No reaction occurred even in the presence of AcOH (10 mol%), Sc(OTf)3 (10 mol%), or TFA (20 mol%). These results strongly indicated that the presence of carboxylic group connected to the alkene at C-3 position was crucial and irreplaceable for this tandem process. Presumably, the carboxyl group in C-3 position of 4-oxo-4H-chromene-3-carboxylic acid 1 can not only render the alkene electron deficient, but also bring the 2-alkyl-azaarene closer to the Michael acceptor by hydrogen bonding.
On the basis of the above-mentioned reactions, the proposed mechanism is outlined in Scheme 4. Basically, 4-oxo-4H-chromene-3-carboxylic acid 1 and 2-methylazaarenes 2 aggregate together via hydrogen-bonding interaction, followed by protonation of 2-methylazaarene 1 to give pyridinium A. As a result of the enhanced acidity of the benzylic proton, the cleavage of benzylic C–H bond ensues to afford enamine intermediate B, which attacks the electron-deficient alkene of 1 via the well-organized transition state I, giving rise to the intermediate D. In the transition state I, hydrogen-bonding interaction between intermediate B and 4-oxo-4H-chromene-3-carboxylic acid 1 helps the nucleophilic enamine B to approach electrophilic alkene moiety. The subsequent decarboxylation and isomerization of intermediate D leads to the final product 3a. The overall result of this tandem process is that the comparatively inert benzylic sp3 C–H bond is activated and directly functionalized. In the whole process, hydrogen bonding plays a key role for the success of this transformation.
Conclusions
In summary, a facile catalyst-free tandem sp3 C–H functionalization/decarboxylation of 4-oxo-4H-chromene-3-carboxylic acid with 2-alkylazaarenes was developed, which efficiently constructed biologically significant azaarene 2-substituted chromanone derivatives in one step. A variety of electronically and sterically diverse azaarenes were well tolerated, and the coupled products were isolated in moderate to good yields. This method proves to be efficient and innovative, which broadens the library of chromanones in pharmaceutical chemistry and extends the synthetic utility of sp3 C–H functionalization in organic synthesis.Acknowledgements
We are grateful to the National Natural Science Foundation of China (no. 21102142) and the Outstanding Young Scientist Award Foundation of Shandong Province (no. BS2011YY007, BS2013YY002) and Qingdao Special Research Foundation of Science and Technology (14-2-4-70-jch). Financial supports from Talents of High Level Scientific Research Foundation (no. 631223) of Qingdao Agricultural University is also gratefully acknowledged.Notes and references
- (a) F. Cottiglia, B. Dhanapal, O. Sticher and J. Heilmann, J. Nat. Prod., 2004, 67, 537 CrossRef CAS PubMed; (b) X. Yang, Y. Sun, Q. Xu and Z. Guo, Org. Biomol. Chem., 2006, 4, 2483 RSC; (c) P. L. Zhao, J. Li and G. F. Yang, Bioorg. Med. Chem., 2007, 15, 1888 CrossRef CAS PubMed; (d) N. Hoettecke, S. Rotzoll, U. Albrecht, M. Lalk, C. Fischer and P. Langer, Bioorg. Med. Chem., 2008, 16, 10319 CrossRef CAS PubMed; (e) P. Y. Chen, Y. C. Kuo, C. H. Chen, Y. H. Kuo and C. K. Lee, Molecules, 2009, 14, 1789 CrossRef CAS PubMed; (f) H. Kikuchi, M. Isobe, M. Sekiya, Y. Abe, T. Hoshikawa, K. Ueda, S. Kurata, Y. Katou and Y. Oshima, Org. Lett., 2011, 13, 4624 CrossRef CAS PubMed; (g) X. Wu, M. Z. Alam, L. Feng, L. S. Tsutsumi, D. Sun and J. G. Hurdle, J. Appl. Microbiol., 2014, 116, 23 CrossRef CAS PubMed; (h) L. F. Tietze, S. Jackenkroll, J. Hierold, L. Ma and B. Waldecker, Chem.–Eur. J., 2014, 20, 8628 CrossRef CAS PubMed; (i) P. N. Moquist, T. Kodama and S. E. Schaus, Angew. Chem., Int. Ed., 2010, 49, 7096 CrossRef CAS PubMed.
- Z.-Q. Xu, J. R. W. Buckheit, T. L. Stup, M. T. Flavin, A. Khilevich, J. D. Rizzo, L. Lin and D. E. Zembower, Bioorg. Med. Chem. Lett., 1998, 8, 2179 CrossRef CAS PubMed.
- G. Sudhakar, S. Bayya, V. D. Kadam and J. B. Nanubolu, Org. Biomol. Chem., 2014, 12, 5601 CAS.
- (a) N. X. Wang, Y. Xing and Y. J. Wang, Curr. Org. Chem., 2013, 17, 1555 CrossRef CAS; (b) A. E. Nibbs and K. A. Scheidt, Eur. J. Org. Chem., 2012, 449 CrossRef CAS PubMed; (c) L. A. Stubbing, F. F. Li, D. P. Furkert, V. E. Caprio and M. A. Brimble, Tetrahedron, 2012, 68, 6948 CrossRef CAS; (d) D. Enders, K. Breuer and J. Runsink, Helv. Chim. Acta, 1996, 79, 1899 CrossRef CAS; (e) J. R. d. Alaniz and T. Rovis, J. Am. Chem. Soc., 2005, 127, 6284 CrossRef PubMed; (f) L. R. Domingo, R. J. Zaragoza, J. A. Saez and M. Arno, Molecules, 2012, 17, 1335 CrossRef CAS PubMed; (g) J. He, J. Zheng, J. Liu, X. She and X. Pan, Org. Lett., 2006, 8, 4637 CrossRef CAS PubMed; (h) S.-L. You, Z.-Q. Rong, Y. Li and G.-Q. Yang, Synlett, 2011, 2011, 1033–1037 CrossRef; (i) A. Gaspar, M. J. Matos, J. Garrido, E. Uriarte and F. Borges, Chem. Rev., 2014, 114, 4960 CrossRef CAS PubMed.
- (a) C. Vila, V. Hornillos, M. Fañanás-Mastral and B. L. Feringa, Chem. Commun., 2013, 49, 5933 RSC; (b) M. K. Brown, S. J. Degrado and A. H. Hoveyda, Angew. Chem., Int. Ed., 2005, 44, 5306 CrossRef CAS PubMed; (c) Ö. Z. Sirin, O. Demirkol, D. Akbaslar and E. S. Giray, J. Supercrit. Fluids, 2013, 81, 217 CrossRef; (d) B. Appel, N. N. Saleh and P. Langer, Chem.–Eur. J., 2006, 12, 1221 CrossRef CAS PubMed; (e) S. J. Coutts and T. W. Wallace, Tetrahedron Lett., 1987, 28, 5195 CrossRef CAS.
- (a) J. R. Zimmerman, M. Manpadi and R. Spatney, Green Chem., 2011, 13, 3103 RSC; (b) Y.-J. Jang, M.-C. Yan, Y.-F. Lin and C.-F. Yao, J. Org. Chem., 2004, 69, 3961 CrossRef CAS PubMed.
- (a) Y. Wei, J. Tang, X. Cong and X. Zeng, Green Chem., 2013, 15, 3165 RSC; (b) C.-H. Tseng, R.-W. Lin, Y.-L. Chen, G.-J. Wang, M.-L. Ho and C.-C. Tzeng, J. Med. Chem., 2011, 54, 3103 CrossRef CAS PubMed.
- (a) M. M. Biddle, M. Lin and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 3830 CrossRef CAS PubMed; (b) A. K. Ghosh, X. Cheng and B. Zhou, Org. Lett., 2012, 14, 5046 CrossRef CAS PubMed.
- C. S. López, R. Erra-Balsells and S. M. Bonesi, Tetrahedron Lett., 2010, 51, 4387 CrossRef.
- E. Jijy, P. Prakash, M. Shimi, P. M. Pihko, N. Joseph and K. V. Radhakrishnan, Chem. Commun., 2013, 49, 7349 RSC.
- T.-L. Liu, Z.-L. He and C.-J. Wang, Chem. Commun., 2011, 47, 9600 RSC.
- Z. Mao, A. Lin, Y. Shi, H. Mao, W. Li, Y. Cheng and C. Zhu, J. Org. Chem., 2013, 78, 10233 CrossRef CAS PubMed.
- (a) M. C. Bagley, C. Glover and E. A. Merritt, Synlett, 2007, 2459 CrossRef CAS; (b) G. D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef CAS; (c) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC.
- (a) B. Qian, S. Guo, C. Xia and H. Huang, Adv. Synth. Catal., 2010, 352, 3195 CrossRef CAS; (b) H. Komai, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2011, 13, 1706 CrossRef CAS PubMed; (c) M. Kanai, S. Matsunaga, H. Komai and T. Yoshino, Synthesis, 2012, 44, 2185–2194 CrossRef.
- (a) J.-J. Jin, H.-Y. Niu, G.-R. Qu, H.-M. Guo and J. S. Fossey, RSC Adv., 2012, 2, 5968 RSC; (b) M. Rueping and N. Tolstoluzhsky, Org. Lett., 2011, 13, 1095 CrossRef CAS PubMed; (c) F.-F. Wang, C.-P. Luo, G. Deng and L. Yang, Green Chem., 2014, 16, 2428 RSC; (d) Z. Q. Zhu, P. Bai and Z. Z. Huang, Org. Lett., 2014, 16, 4881 CrossRef CAS PubMed.
- (a) J. Y. Liu, H. Y. Niu, S. Wu, G. R. Qu and H. M. Guo, Chem. Commun., 2012, 48, 9723 RSC; (b) B. Qian, S. Guo, J. Shao, Q. Zhu, L. Yang, C. Xia and H. Huang, J. Am. Chem. Soc., 2010, 132, 3650 CrossRef CAS PubMed.
- (a) S. J. Lou, D. Q. Xu, D. F. Shen, Y. F. Wang, Y. K. Liu and Z. Y. Xu, Chem. Commun., 2012, 48, 11993 RSC; (b) P. X. Qian, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef PubMed; (c) Y. Li, F. Guo, Z. Zha and Z. Wang, Chem.–Asian J., 2013, 8, 534 CrossRef CAS PubMed.
- (a) R. Niu, S. Yang, J. Xiao, T. Liang and X. Li, Chin. J. Catal., 2012, 33, 1636 CrossRef CAS; (b) V. B. Graves and A. Shaikh, Tetrahedron Lett., 2013, 54, 695 CrossRef CAS.
- (a) F. F. Wang, C. P. Luo, Y. Wang, G. Deng and L. Yang, Org. Biomol. Chem., 2012, 10, 8605 RSC; (b) S. V. N. Vuppalapati and Y. R. Lee, Tetrahedron, 2012, 68, 8286 CrossRef CAS; (c) A. Kumar, L. P. Gupta and M. Kumar, RSC Adv., 2013, 3, 18771 RSC; (d) H.-Y. Li, L.-J. Xing, T. Xu, P. Wang, R.-H. Liu and B. Wang, Tetrahedron Lett., 2013, 54, 858 CrossRef CAS; (e) H. M. Meshram, N. Nageswara Rao, L. Chandrasekhara Rao and N. Satish Kumar, Tetrahedron Lett., 2012, 53, 3963 CrossRef CAS; (f) K. Kumari, B. K. Allam and K. N. Singh, RSC Adv., 2014, 4, 19789 RSC; (g) M. Raghu, M. Rajasekhar, B. Chandra Obula Reddy, C. Suresh Reddy and B. V. Subba Reddy, Tetrahedron Lett., 2013, 54, 3503 CrossRef CAS; (h) X. Gao, F. Zhang, G. Deng and L. Yang, Org. Lett., 2014, 16, 3664 CrossRef CAS PubMed.
- (a) R. Niu, J. Xiao, T. Liang and X. Li, Org. Lett., 2012, 14, 676 CrossRef CAS PubMed; (b) L. Xu, Z. Shao, L. Wang and J. Xiao, Org. Lett., 2014, 16, 796 CrossRef CAS PubMed; (c) Z. Shao, L. Xu, L. Wang, H. Wei and J. Xiao, Org. Biomol. Chem., 2014, 12, 2185 RSC; (d) J. Xiao, K. Zhao and T. P. Loh, Chem. Commun., 2012, 48, 3548 RSC; (e) T. Liang, J. Xiao, Z. Xiong and X. Li, J. Org. Chem., 2012, 77, 3583 CrossRef CAS PubMed; (f) X. Yu, S. Yu, J. Xiao, B. Wan and X. Li, J. Org. Chem., 2013, 78, 5444 CrossRef CAS PubMed; (g) X. Li, Y. Jiang, X. Wang, X. Li and J. Xiao, Synlett, 2012, 23, 1649–1652 CrossRef; (h) J. Xiao, Y. Chen, S. Zhu, L. Wang, L. Xu and H. Wei, Adv. Synth. Catal., 2014, 356, 1835–1845 CrossRef CAS; (i) L. Wang, J. Xiao and T.-P. Loh, ChemCatChem, 2014, 6, 1183 CAS; (j) F. Huang, L. Xu and J. Xiao, Chin. J. Chem., 2012, 30, 2721 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09338k |
| This journal is © The Royal Society of Chemistry 2014 |