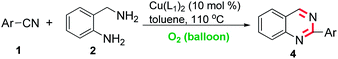Copper-catalyzed intermolecular cyclization of nitriles and 2-aminobenzylamine for 3,4-dihydroquinazolines and quinazolines synthesis via cascade coupling and aerobic oxidation†
Cheng Li‡
,
Shujuan An‡,
Yuelu Zhu,
Jin Zhang,
Yifan Kang,
Ping Liu*,
Yaoyu Wang and
Jianli Li*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of The Ministry of Education and College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi 710069, P. R. China. E-mail: lijianli@nwu.edu.cn; liuping@nwu.edu.cn; Fax: +86 2988308396
First published on 1st October 2014
Abstract
A copper-catalyzed cascade coupling and aerobic oxidation of aromatic nitriles and 2-aminobenzylamine for the synthesis of 3,4-dihydroquinazolines and quinazolineshas been achieved. The catalytic system, characterized by not adding additives and using molecular oxygen as the sole oxidant, exhibited exquisite substrate specificity and operated under mild conditions through inherently “green” processes.
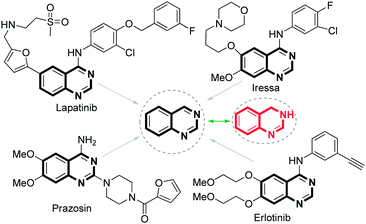
Among pharmaceutical substances, the N-heterocycles comprise an important class.1 A recent survey found that more than 70% of the top-selling proprietary drugs contain N-heterocycles in their structures.2 As such, 2-substituted quinazolines and 3,4-dihydroquinazolines have been widely used in organic synthesis and medicinal chemistry as building blocks because of their biological and therapeutic activities (Fig. 1).3,4 For example, quinazolines are used as ligands for benzodiazepine and GABA receptors in the CNS system5 or as DNA binders.6 There are some synthetic strategies available for the synthesis of quinazolines7,8 involving several substrates such as 2-halophenylmethanamines9 or 2-aminobenzoketones10 or amidines11 or 2-aminobenzaldehydes12 (Scheme 1). Despite these advances, the reported synthetic approachs suffer from some drawbacks that nonrenewable oxidant has to be used and the yields are not satisfactory. Unfortunately, few methods are reported for synthetic approaches to 3,4-dihydroquinazolines until now,11a,13 and this transformation is considered to be a big challenge. Therefore, novel and efficient methods for the synthesis of 2-substituted quinazolines and 3,4-dihydroquinazolines that are compatible with various functional groups and proceed under mild conditions remain highly desirable. Herein, we report the transformations of various nitriles with 2-aminobenzylamine via copper-catalyzed cascade coupling to synthesize 3,4-dihydro-quinazolines and aerobic oxidative process to produce 2-substituted quinazolines. The catalytic system, characterized by not adding additives and using molecular oxygen as the sole oxidant, exhibited exquisite substrate specificity and operate under mild conditions through inherently “green” processes. Particularly, the present method could provide a practical route for synthesis of 3,4-dihydroquinazolines for the first time.
To standardize the reaction conditions a series of experiments were performed with variation of reaction parameters such as catalyst, solvent and temperature for are presentative coupling of benzonitrile 1a and 2-aminobenzylamine 2. As shown in Table 1, screening of various copper catalysts (entries 1–9) revealed that CuII(L1)2 displayed greater catalytic reactivity for the transformations (entry 6). Only a trace amount of target product was observed in the absence of copper catalyst (entry 10). Optimization of different solvents revealed that EtOH, dioxane and CH3CN were inferior to toluene in the reaction (entries 11–13). There action temperature was also varied, and 110 °C gave the best result (compare entries 14–16). Accordingly, the optimized reaction conditions are as follows: CuII(L1)2 (10 mol%), under air atmosphere in toluene at 110 °C.
| Entry | Catalyst | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a The reaction conditions were as follow: a mixture of benzonitrile (1a, 1 mmol), 2-aminobenzylamine (2, 1.25 mmol), catalyst (0.1 mmol), solvent (2 mL) were heated in a 10 mL flask for 12 h under air.b Yield of the isolated pure product.c n.d. = not detected. | ||||
| 1 | CuCl2 | EtOH | 75 | n.d. |
| 2 | CuBr2 | EtOH | 75 | n.d. |
| 3 | CuI2 | EtOH | 75 | n.d.c |
| 4 | Cu(OAc)2 | EtOH | 75 | 20 |
| 6 | CuII(L1)2 | EtOH | 75 | 60 |
| 7 | CuII(L2)2 | EtOH | 75 | <5 |
| 8 | CuII(L3)2 | EtOH | 75 | 13 |
| 9 | CuII(L4)2 | EtOH | 75 | 38 |
| 10 | — | EtOH | 75 | n.d. |
| 11 | CuII(L1)2 | CH3CN | 75 | 62 |
| 12 | CuII(L1)2 | Toluene | 75 | 65 |
| 13 | CuII(L1)2 | Dioxane | 75 | 55 |
| 14 | CuII(L1)2 | Toluene | 90 | 72 |
| 15 | CuII(L1)2 | Toluene | 100 | 75 |
| 16 | CuII(L1)2 | Toluene | 110 | 81 |
With the optimized reaction conditions established, the scope and generality of the reaction were investigated (Table 2). There action showed high functional group tolerance and proved to beaquitegeneral method for the preparation of dihydroquinazoline 3. In general, the electron-deficient aromatic nitriles showed better reactivity and provided higher yields than electron-rich ones, and the reactions were insensitive to the steric hindrance of the substituents on the aromatic rings. The nitriles possessing phenyl, pyridyl, and thienyl groups underwent the desired reaction to give the corresponding dihydroquinazolines 3a–3e in good yields. However, 2-(pyridin-3-yl)-3,4-dihydroquinazoline 3c was obtained in trace amounts due to the volatility of dihydroquinazoline. Nitriles with fluoro, chloro, bromo, nitro, and trifluoromethyl groups on the phenyl ring reacted smoothly to give the corresponding dihydroquinazolines 3f–3o in excellent yields in the reactions. The ability to incorporate C–Br and C–Cl bonds makes this method appealing, since it offers an opportunity for further transformation. An extensive investigation of the reaction showed that dinitriles were converted into the respective monodyhydroquinazolines with excellent chemoselectivity (3p–3s). It is note worthy that mixtures of dinitriles (1 mmol) with double 2-aminobenzylamine (2.5 mmol) gave only 2-substitutedmonodihydroquinazolines, which is of practical significance because the remaining nitrile group can be converted into other important functional groups. Even if sterically bulky aromatic nitriles such as 3,4,5,6-tetrafluorophthalonitrile 1s was used, the corresponding 3s were obtained in 89% yields. Unfortunately, couplings of 2-aminobenzylamine with alkyl nitriles did not work by using a similar procedure.
Next, we continued our investigation by studying the conversion of 1 and 2 to the desired quinazolines 4 using a combination of a copper catalyst together with O2 as the sole oxidant (Table 3). Satisfactorily, quinazolines could be obtained smoothly with good results. These results that showed electron-withdrawing groups had more favorable effects than electron-donating groups. As is depicted in Table 3, nitriles with fluoro, chloro, nitro, and trifluoromethyl groups on the phenyl ring reacted smoothly to give the corresponding products in high yields. These functional groups provide ample opportunity for further synthetic manipulations. The introduction of fluorine atoms or fluorine-containing groups into heterocyclic rings has made possible the discovery of new bioactive products. We then succeeded in performing the reaction to demonstrate the practicability of the developed method in a one-pot fashion, and our oxidation reaction could provide a useful route for drug discovery. Similarly, copper-catalyzed aerobic oxidation of 2-aminobenzylamine with alkyl nitrile was not effective under this set of conditions.
To gain more insight into the mechanism of the reaction, when 3a was treated under oxygen atmosphere for 6 h, 98% yield of aromatized quinazoline 4a could be obtained. However, when 3a was treated in the absence of CuII(L1)2, 4a was obtained in 42% yield (Scheme 2). This result indicated that the 3,4-dihydroquinazolines 3 was probably an intermediate in the synthesis of quinazoline 4, and the copper catalyst serves a double catalytic function for both cascade coupling and aerobic oxidation.
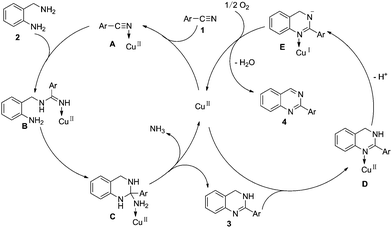
A tentative mechanism is depicted in Scheme 3. First, the nitrile 1 is activated by CuII(L1)2 to give intermediate A.14 Nucleophilic addition of A with 2-aminobenzylamine 2 provides B. Intramolecular cycloaddition of B provides C, intermediate C releases CuII(L1)2 and NH3 to afford 3. As part of our ongoing effort, we also calculate the Fukui function (f+) of the compound 3 (Fig. 2), the result proved the tentative mechanism. The nitrogen atom of tertiary amines 3 coordinate to CuII(L1)2 to give D by a ligand-exchange reaction. Then the intermediate D through deprotonation to form E. Finally, after deprotonation would liberate quinazolines 4 and the reoxidation of the Cu(I) complex to the Cu(II) complex would close the catalytic cycle.15
Conclusions
In summary, we have developed a novel and efficient method for synthesis of 3,4-dihydroquinazolines and quinazolines from aromatic nitriles and 2-aminobenzylamine via copper-catalyzed cascade coupling and aerobic oxidation without addition of any base or additive. This method features demonstrated that: (1) the copper catalyst serves double catalytic function for both cascade coupling and aerobic oxidation; (2) the use of oxygen as the terminal oxidant and the one-pot method can be employed for the reactions. Further studies on the scope of the substrates, applications, and the precise mechanism are underway in our laboratory.Acknowledgements
We thank the National Natural Science Foundation of China (NSFC 21272184; 20972124 and 21143010) and the Shaanxi Provincial Natural Science Fund Project (no. 2012JQ2007) for financial support.Notes and references
- (a) E. Kleemann and R. Kutscher, Pharmaceutical Substances, Thieme, Stuttgart, 2009 Search PubMed; (b) V. Alagarsamy, V. R. Solomon and K. Dhanabal, Bioorg. Med. Chem., 2007, 15, 235 CrossRef CAS PubMed.
- (a) M. G. Bursavich, D. Dastrup, M. Shenderovich, K. M. Yager, D. M. Cimbora, B. Williams and D. V. Kumar, Bioorg. Med. Chem. Lett., 2013, 23, 6829 CrossRef CAS PubMed; (b) Y. Zhang, M. R. Sheets, E. K. Raja, K. N. Boblak and D. A. Klumpp, J. Am. Chem. Soc., 2011, 133, 8467 CrossRef CAS PubMed.
- (a) B. A. Foster, H. A. Coffrey, M. J. Morin and F. Rastinejad, Science, 1999, 286, 2507 CrossRef CAS PubMed; (b) R. Gundla, R. Kazemi, R. Sanam, R. Muttineni, J. A. R. P. Sarma, R. Dayam and N. Neamati, J. Med. Chem., 2008, 51, 3367 CrossRef CAS PubMed; (c) L. A. Doyle and D. D. Ross, Oncogene, 2003, 2, 7340 CrossRef PubMed; (d) D. W. Fry, A. J. Kraker, A. McMichael, L. A. Ambroso, J. M. Nelson, W. R. Leopold, R. W. Connors and A. J. Bridges, Science, 1994, 265, 1093 CAS.
- (a) A. Luth and W. Lowe, Eur. J. Med. Chem., 2008, 43, 1478 CrossRef PubMed; (b) J. F. Mendes da Silva, M. Walters, S. Al-Damluji and C. R. Ganellin, Bioorg. Med. Chem., 2008, 16, 7254 CrossRef CAS PubMed; (c) H. A. Burris III, Oncologist, 2004, 9, 10 CrossRef.
- (a) V. Colotta, D. Catarzi, F. Varano, O. Lenzi, G. Filacchioni, C. Costagli, A. Galli, C. Ghelardini, N. Galeotti, P. Gratteri, J. Sgrignani, F. Deflorian and S. Moro, J. Med. Chem., 2006, 49, 6015 CrossRef CAS PubMed; (b) A. Lewerenz, S. Hentschel, Z. Vissiennon, S. Michael and K. Nieber, Drug Dev. Res., 2003, 58, 420 CrossRef CAS.
- N. Malecki, P. Carato, G. Rigo, J. F. Goossens, R. Houssin, C. Bailly and J. P. Henichart, Bioorg. Med. Chem., 2004, 12, 641 CrossRef CAS PubMed.
- (a) W. Szczepankiewicz, J. Suwiński and R. Bujok, Tetrahedron, 2000, 56, 9343 CrossRef CAS; (b) V. L. Truong and M. Morrow, Tetrahedron Lett., 2010, 51, 758 CrossRef CAS; (c) M. A. McGowan, C. Z. McAvoy and S. L. Buchwald, Org. Lett., 2012, 14, 3800 CrossRef CAS PubMed; (d) C. C. Malakar, A. Baskakova, J. Conrad and U. Beifuss, Chem.–Eur. J., 2012, 18, 8882 CrossRef CAS PubMed; (e) F. Shibahara, A. Yoshida and T. Murai, Chem. Lett., 2008, 37, 646 CrossRef CAS; (f) A. Chilin, G. Marzaro, S. Zanatta and A. Guiotto, Tetrahedron Lett., 2007, 48, 3229 CrossRef CAS; (g) F. Portela-Cubillo, J. S. Scott and J. C. Walton, Chem. Commun., 2008, 2935 RSC.
- (a) D. S. Yoon, Y. Han, T. M. Stark, J. C. Haber, B. T. Gregg and S. B. Stankovich, Org. Lett., 2004, 6, 4775 CrossRef CAS PubMed; (b) S. Madabhushi, K. Kumar Reddy Mallu, R. Jillella, S. Kurva and R. Singh, Tetrahedron Lett., 2014, 55, 1979 CrossRef CAS; (c) A. Chilin, G. Marzaro, S. Zanatta and A. Guiotto, Tetrahedron Lett., 2007, 48, 3229 CrossRef CAS; (d) D. J. Connolly, D. Cusack, T. P. O'Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS; (e) Y. Bathini, I. Sidhu, R. Singh, R. G. Micetich and P. L. Toogood, Tetrahedron Lett., 2002, 43, 3295–3296 CrossRef CAS.
- (a) C. Wang, S. Li, H. Liu, Y. Jiang and H. Fu, J. Org. Chem., 2010, 75, 7936 CrossRef CAS PubMed; (b) J. Zhang, C. Yu, S. Yang, C. Wan and Z. Wang, Chem. Commun., 2010, 46, 5244–5246 RSC.
- (a) J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS PubMed; (b) B. Han, C. Wang, R. F. Han, W. Yu, X. Y. Duan, R. Fang and X. L. Yang, Chem. Commun., 2011, 47, 7818 RSC; (c) S. Ferrini, F. Ponticelli and M. Taddei, Org. Lett., 2007, 9, 69 CrossRef CAS PubMed; (d) K. Karnakar, A. V. Kumar, S. N. Murthy, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53(34), 4613–4617 CrossRef CAS; (e) J. Zhu, H. Xie, Z. Chen, S. Li and Y. Wu, Chem. Commun., 2011, 47, 1512–1514 RSC.
- (a) Y. Lv, Y. Li, T. Xiong, W. Pu, H. Zhang, K. Sun, Q. Liu and Q. Zhang, Chem. Commun., 2013, 49, 6439 RSC; (b) Y. Ohta, Y. Tokimizu, S. Oishi, N. Fujii and H. Ohno, Org. Lett., 2010, 12, 3963 CrossRef CAS PubMed.
- (a) H. Yuan, W. J. Yoo, H. Miyamura and S. Kobayashi, Adv. Synth. Catal., 2012, 354, 2899 CrossRef CAS; (b) C. U. Maheswari, G. S. Kumar, M. Venkateshwar, R. A. Kumar, M. L. Kantam and K. R. Reddy, Adv. Synth. Catal., 2010, 352, 341 CrossRef CAS; (c) B. Han, X. L. Yang, C. Wang, Y. W. Bai, T. C. Pan, X. Chen and W. Yu, J. Org. Chem., 2012, 77, 1136 CrossRef CAS PubMed; (d) J. J. Vanden Eynde, J. Godin, A. Mayence, A. Maquestiau and E. Anders, Synthesis, 1993, 867 CrossRef CAS; (e) Y. Peng, Y. Zeng, G. Qiu, L. Cai and V. W. Pike, J. Heterocycl. Chem., 2010, 47, 1240 CrossRef CAS; (f) C. Huang, Y. Fu, H. Fu, Y. Jiang and Y. Zhao, Chem. Commun., 2008, 6333 RSC; (g) J. Ju, R. Hua and J. Su, Tetrahedron, 2012, 68, 9364–9370 CrossRef CAS; (h) Z. Chen, J. Chen, M. Liu, J. Ding, W. Gao, X. Huang and H. Wu, J. Org. Chem, 2013, 78, 11342 CrossRef CAS PubMed.
- (a) F. Portela-Cubillo, J. S. Scott and J. C. Walton, J. Org. Chem., 2009, 74, 4934 CrossRef CAS PubMed; (b) R. Sarma and D. Prajapati, Green Chem., 2011, 13, 718 RSC.
- G. Rousselet, P. Capdevielle and M. Maumy, Tetrahedron Lett., 1993, 34, 6395 CrossRef CAS.
- (a) A. Taketoshi, A. Tsujimoto, S. Maeda, T. Koizumi and T. Kanbara, ChemCatChem, 2010, 2, 58–60 CrossRef CAS; (b) J. H. Li and L. Neuville, Org. Lett., 2013, 15, 1752–1755 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, copies of 1HNMR, 13C NMR and IR spectra. CCDC 977533 and 977534. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra09240f |
| ‡ Cheng Li and Shujian An contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |