An efficient synthesis of novel dibenzoxdiazepine-fused heterocycles through a multicomponent reaction†
Abstract
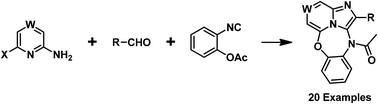
A one-pot synthesis of 6-oxa-2,2a1,11-triazadibenzo[cd,g]azulenes by a three-component reaction of a 2-aminoheterocycle, aldehydes, and 2-isocyanophenyl acetate is presented. This efficient and green protocol has the advantages of environmental friendliness, high yields and operational simplicity. The atropisomeric properties of this unique structure were examined by 1H NMR spectroscopy and X-ray structural analyses, and the barriers to their interconversion were clarified.

 Please wait while we load your content...
Please wait while we load your content...