Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrasound assisted synthesis of a Zn(II) metal–organic framework with nano-plate morphology using non-linear dicarboxylate and linear N-donor ligands†
Mohammad Yaser
Masoomi
a,
Ali
Morsali
*a and
Peter C.
Junk
b
aDepartment of Chemistry, Faculty of Sciences, Tarbiat Modares University, Tehran, Islamic Republic of Iran. E-mail: morsali_a@modares.ac.ir; Tel: +98-21-82883449
bCollege of Science, Technology & Engineering, James Cook University, Townsville, Queensland 4811, Australia
First published on 24th September 2014
Abstract
A 3D, porous Zn(II)-based metal–organic framework {[Zn2(oba)2(4-bpdb)]·2DMF}n (TMU-4) with double interpenetration was prepared by using a non-linear dicarboxylate (H2oba = 4,4′-oxybisbenzoic acid) and a linear N-donor (4-bpdb = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene) ligand. Also micro- and nano-plates of this MOF were synthesized by a sonochemical process and characterized by scanning electron microscopy, X-ray powder diffraction and IR spectroscopy. The thermal stability was studied by thermogravimetric analysis (TGA). Sonication time and concentration of initial reagents effects on the size and morphology of nano-structured MOFs, were studied. Calcination of TMU-4 at 500 °C under air atmosphere yields ZnO nanoparticles.
Introduction
Three-dimensional (3D) metal–organic frameworks (MOFs) are a new class of porous materials which are highly attractive because of their potential use as functional materials in structure-dependent applications, such as gas storage and separation, ion exchange, sensing, catalysis, and drug delivery.1–8 The MOFs can be designed by choosing appropriate organic ligands and inorganic secondary building units (SBUs).9Of the many ligands that have been employed for the preparation of MOF structures, using a combination of functionalized dicarboxylic acids and N-donor ligands can lead to MOFs with desired properties.5,10,11 Meanwhile, there is also an increasing interest in MOFs with flexible and dynamic frameworks with appropriate groups in their structures to exhibit high selectivity for guest inclusion and structural transformation upon adsorption/desorption of guest molecules.12,13
Reducing the size of MOFs to nanoscale has been extremely attractive.4 Recently using ultrasound irradiation in synthesis and preparation of nano or microstructures of MOFs has been of interest.14–17 In the research area of sonochemistry molecules undergo a chemical reaction because of the application of powerful ultrasound radiation (20 KHz–10 MHz)18 which induce chemical or physical changes during cavitation. Cavitation involves formation, growth, and instantaneously implosive collapse of bubbles in a liquid, which generates local hot spots having temperatures up to 5000 °C, 500 atm pressures with a lifetime of a few microseconds.19–21 These extreme conditions can also enhance the formation of nano-sized structures, mostly via an increase of crystallization nuclei.22
ZnO is a polar inorganic material and an important n-type semiconductor with a wide band gap energy of 3.37 eV,23 and is an excellent material for potential applications including solar cells, luminescent materials, transparent conductors and gas sensors.24–28 Up until now, various synthetic methods have been developed to produce zinc oxide nanostructures.29,30 Thermal decomposition of MOFs under various conditions has been widely studied for the preparation of ZnO nanostructures with different sizes and morphologies.31
In this work, we have synthesized a Zn(II) metal–organic framework based on a V-shaped flexible dicarboxylate ligand, 4,4′-oxybis(benzoic acid) (H2oba) and the N-donor ligand 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene (4-bpdb) and investigated the effect of ultrasonic irradiation on shape and size. Also TMU-4 was calcined at 500 °C to prepare ZnO nanoparticles.
Experimental
Materials and physical techniques
All reagents for the synthesis and analysis were commercially available from Aldrich and Merck Company and used as received. The ligand 4-bpdb (1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene) was prepared by the reported method.32 Melting points were measured on an Electrothermal 9100 apparatus. IR spectra were recorded using Thermo Nicolet IR 100 FT-IR.Ultrasonic generation was carried out in an ultrasonic bath SONICA-2200 EP (frequency of 40 KHz). The samples were characterized with a field emission scanning electron microscope (FE-SEM) Philips XL30 and TESCAN MIRA (Czech) with gold coating.
The thermal behaviour was measured with a PL-STA 1500 apparatus with the rate of 10 °C min−1 in a static atmosphere of argon. X-ray powder diffraction (XRD) measurements were performed using a Philips X'pert diffractometer with mono chromated Cu-Kα radiation.
Single crystals of TMU-4 were coated with viscous hydrocarbon oil and mounted on a loop. Data were obtained at −173 °C (100 K) on the MX1: Macromolecular Crystallography beamline at the Australian Synchrotron, Victoria, Australia. Data collection and integration on the MX1: Macromolecular Crystallography beamline were carried out using Blu-Ice33 and the XDS software package.34 The structure was solved using SHELXS35 and refined by full-matrix least-squares on all F2 data using SHELX,35 in conjunction with the X-Seed graphical user interface.36 All hydrogen atoms were placed in calculated positions using the riding model. Sorption study on TMU-4 was performed using the AutosorbIQ from Quantachrome Instruments: CO2 at 195 K. The sample was activated at 140 °C for 12 hours under vacuum. Dynamic light scattering measurements of particle sizes were determined by means of a Zetasizer Nano equipment.
Synthesis of {[Zn2(oba)2(4-bpdb)]·2DMF}n (TMU-4)
| Samples name | Molar ratio (oba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-bpdb 4-bpdb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn(OAc)2) mmol in 25 mL DMF Zn(OAc)2) mmol in 25 mL DMF |
Concentration [oba]/[4 bpdb]/[Zn(OAc)2] (M) | Time (min) | Elemental analysis, founda (%) | Yield (%) | Morphology |
|---|---|---|---|---|---|---|
| a Calculated for [Zn2(C14O5H8)2(C12H10N4)](C3NOH7)2: C: 55.3, H: 4.0, N: 8.4. | ||||||
| A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
[0.04]/[0.04]/[0.024] | 30 | C: 55.8, H: 3.7, N: 8.4 | 75 | Micro plate |
| B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
[0.04]/[0.04]/[0.024] | 60 | C: 55.6, H: 3.8, N: 8.1 | 80 | Micro plate |
| C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
[0.04]/[0.04]/[0.024] | 90 | C: 55.4, H: 3.9, N: 8.2 | 85 | Micro plate |
| D | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
[0.06]/[0.06]/[0.036] | 90 | C: 55.6, H: 3.9, N: 8.5 | 82 | Micro plate |
| E | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
[0.02]/[0.02]/[0.012] | 90 | C: 55.9, H: 3.8, N: 8.3 | 88 | Micro plate |
| F | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
[0.02]/[0.02]/[0.012], TEA = 2 mL, pH = 6 | 90 | C: 55.4, H: 3.7, N: 8.4 | 56 | Nano plate |
| G | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
[0.02]/[0.02]/[0.012], TEA = 3 mL, pH = 7 | 90 | C: 55.2, H: 3.6, N: 8.0 | 51 | Nano plate |
Results and discussion
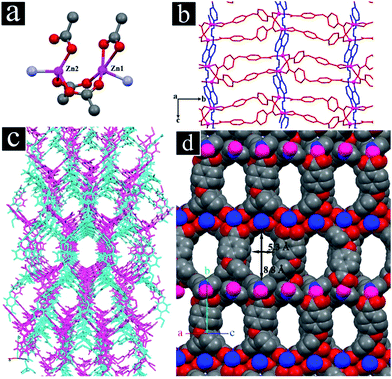
Yellow crystals of TMU-4 were synthesized by mixing H2oba, Zn(NO3)2·6H2O and 4-bpdb in DMF solvent at 80 °C for 3 days. Single-crystal X-ray diffraction reveals that TMU-4 crystallized in the space group P21/c with the formula of {[Zn2(oba)2(4-bpdb)]·2DMF}n.37 TMU-4 is based on a binuclear Zn2 unit (Zn#1 and Zn#2), both of which have tetrahedral geometry and coordinated to three carboxylate O atoms (for Zn#1: O2, O7, O10, and for Zn#2: O1, O5, O6) from three adjacent oba ligands and one N atom (Zn#1: N4 and Zn#2: N1) from the 4-bpdb ligand (Fig. 1a). The distance between Zn#1 and Zn#2 is 3.358 Å. Each non-linear (C–O–C = 118.0(6)°) dicarboxylate oba ligand binds three consecutive Zn(II) centers from two different units: one carboxylate group of an oba ligand adopts a bidentate mode and bridges both Zn#1 and Zn#2 centers of the unit while the other one is monodentate and binds to either Zn#1 or Zn#2, thereby forming 2D sheets (Fig. 1b). The 2D sheets are connected through the linear 4-bpdb, extending the structure in three dimensions. The V-shaped coordination of the oba ligand plays an important role in the linkage of the nodes resulting in a three-dimensional honeycomb framework with double interpenetration (Fig. 1c). Although the double interpenetration is formed to avoid an extremely large void space, but still possesses one-dimensional (1D) open channels (aperture size: 5.3 × 8.8 Å, taking into account the van der Waals radii; 35.9% void space per unit cell)38 running along the [101] direction and lattice DMF molecules are accommodated in the channels (Fig. 1d). TMU-4 is porous to CO2 at 195 K and 1 bar (148.90 cm3 g−1 at 1 bar; BET surface area calculated over p/p0 = 0.02–0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.9 m2 g−1) (Fig. S2, ESI†). The pore volume calculated from the CO2 adsorption is 0.298 cm3 g−1.39
517.9 m2 g−1) (Fig. S2, ESI†). The pore volume calculated from the CO2 adsorption is 0.298 cm3 g−1.39
The morphology and size of TMU-4 were investigated using scanning electron microscopy (SEM) by changing two parameters; sonication time and concentration of starting materials as well as control of nucleation.
Firstly, sonication time as a parameter was changed at constant concentration of [0.04] M of starting materials. In all three different times (30, 60 and 90 min) micro-plates of TMU-4 were obtained in which higher sonication time (90 min) leads to uniform distribution in plate size (Fig. 2 and S3, ESI†). Hence, sonication time of 90 min is considered as the optimized value.
 | ||
| Fig. 2 FE-SEM images of nano-plates of TMU-4 synthesized by sonochemical reaction (a) sample A, (b) sample B and (c) sample C. | ||
After this in order to investigate the role of concentration of initial reagents on the morphology and size of product, reactions were performed with two different concentrations of starting materials ([0.06] M and [0.02] M). The results show that high concentrations of initial reagents increased particle size and thus lower concentration of initial reagents reduced the size of plates (Fig. 3). After this, increasing the speed of nucleation during the synthesis of TMU-4 was tested by adding triethylamine (TEA). Using TEA causes fast nucleation of product due to deprotonated oba ligand and faster nucleation reduces particle size. In this mechanism nano-plates with a narrow size distribution were obtained (Fig. 3c and d).22 By this, width of nano-plate reduces to 90 nm (Fig. 3d).
 | ||
| Fig. 3 FE-SEM images of nano-plates of TMU-4 synthesized by sonochemical reaction (a) sample D, (b) sample E, (c) sample F and (d) sample G. | ||
The IR spectra of both crystals produced by conventional heating and nano-plate produced by the sonochemical method show the symmetric νsym (COO) and asymmetric νas (COO) vibrations of the carboxylate groups at 1409 cm−1 and 1604 cm−1, respectively. Also the characteristic absorption peak (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O = 1679 cm−1) of DMF are presented in the IR spectra of TMU-4 (Fig. S4, ESI†).
O = 1679 cm−1) of DMF are presented in the IR spectra of TMU-4 (Fig. S4, ESI†).
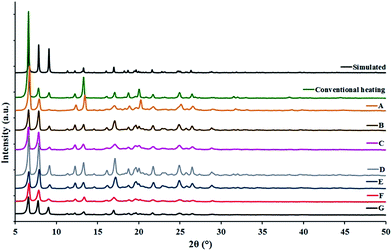
A comparison between powder X-ray diffraction (PXRD) patterns of the simulated (derived from the single crystal structure of TMU-4) and experimental (resulting from the sonochemical process) confirms that the sonochemically synthesized TMU-4 is structurally identical to TMU-4 prepared through conventional heating (Fig. 4).
To examine the thermal stability of TMU-4 thermogravimetric analysis (TGA) was carried out between 25 and 600 °C. The TGA curve of TMU-4 (conventional heating) shows a plateau in the range of 25 to 100 °C followed by a continuous loss of 14.5% (expected: 14.6%) up to 305 °C, which can be ascribed to removal of the guest DMF molecules (Fig. 5).
Decomposition of TMU-4's framework occurs in the temperature range of 305–500 °C. Final residual mass 18.4% (expected: 19%) and the XRD pattern of the final decomposition product show the formation of ZnO (Fig. S5, ESI†).
To prepare zinc oxide nanoparticles, TMU-4 was calcined at 500 °C for 2 h. Fig. S5† depicts the XRD patterns of the residue obtained from calcination of TMU-4. The Bragg diffraction peaks in the range of 2θ = 20–80° exhibit the typical patterns of hexagonal wurtzite structure of ZnO consistent with the reported data by the JCPDS card number 36-1451 with lattice parameters of a = 3.25 and c = 5.20 Å. The SEM image of the residue obtained from the direct calcination of TMU-4 at 500 °C shows the formation of ZnO nanoparticle (Fig. 6).
Conclusions
A 3D, porous metal–organic framework {[Zn2(oba)2(4-bpdb)]·2DMF}n (TMU-4) with double interpenetration was synthesized by conventional heating and analysed by X-ray crystallography. Using ultrasonic irradiation leads to formation of micro- and nano-plates of TMU-4 that were characterized by scanning electron microscopy, X-ray powder diffraction and IR spectroscopy. To prepare the nanostructure of TMU-4, three different times and concentrations of initial reagents, [0.06], [0.04] and [0.02] M, were tested. Also the rate of nucleation was tested by adding TEA to the reaction. Results show that best uniform distribution of nano-plates TMU-4 were obtained in 90 min with concentration of [0.02] M in the presence of TEA. These results indicate that sonochemical process can be used as an effective method for fast and readily preparation of nano-MOFs. Also calcination of TMU-4 at 500 °C under air atmosphere yields ZnO nanoparticles.Acknowledgements
Support of this investigation by Tarbiat Modares University is gratefully acknowledged.Notes and references
- E. Lallana, A. Sousa-Herves, F. Fernandez-Trillo, R. Riguera and E. Fernandez-Megia, Pharm. Res., 2012, 29, 1–34 CrossRef CAS PubMed
.
- Z. Ma and B. Moulton, Coord. Chem. Rev., 2011, 255, 1623–1641 CrossRef CAS
.
- M. Y. Masoomi and A. Morsali, Coord. Chem. Rev., 2012, 256, 2921–2943 CrossRef CAS
.
- M. Y. Masoomi and A. Morsali, RSC Adv., 2013, 3, 19191–19218 RSC
.
- A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed
.
- M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2011, 112, 782–835 CrossRef PubMed
.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2011, 112, 1105–1125 CrossRef PubMed
.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2011, 112, 1232–1268 CrossRef PubMed
.
- N. Stock and S. Biswas, Chem. Rev., 2011, 112, 933–969 CrossRef PubMed
.
- R. Kitaura, K. Seki, G. Akiyama and S. Kitagawa, Angew. Chem., Int. Ed., 2003, 42, 428–431 CrossRef CAS PubMed
.
- P. Mahata, M. Prabu and S. Natarajan, Cryst. Growth Des., 2009, 9, 3683–3691 CAS
.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Science, 2010, 330, 650–653 CrossRef CAS PubMed
.
- K. C. Stylianou, J. E. Warren, S. Y. Chong, J. Rabone, J. Bacsa, D. Bradshaw and M. J. Rosseinsky, Chem. Commun., 2011, 47, 3389–3391 RSC
.
- N. Soltanzadeh and A. Morsali, Ultrason. Sonochem., 2010, 17, 139–144 CrossRef CAS PubMed
.
- M. J. S. Fard-Jahromi and A. Morsali, Ultrason. Sonochem., 2010, 17, 435–440 CrossRef CAS PubMed
.
- A. Aslani and A. Morsali, Inorg. Chim. Acta, 2009, 362, 5012–5016 CrossRef CAS
.
- M. Y. Masoomi, G. Mahmoudi and A. Morsali, J. Coord. Chem., 2010, 63, 1186–1193 CrossRef CAS
.
- K. S. Suslick, S.-B. Choe, A. A. Cichowlas and M. W. Grinstaff, Nature, 1991, 353, 414–416 CrossRef CAS
.
- R. Feng, Y. Zhao, C. Zhu and T. J. Mason, Ultrason. Sonochem., 2002, 9, 231–236 CrossRef CAS PubMed
.
- M. Strasberg, J. Acoust. Soc. Am., 1959, 31, 163–176 CrossRef
.
- K. Negishi, J. Phys. Soc. Jpn., 1961, 16, 1450–1465 CrossRef CAS
.
- D. Tanaka, A. Henke, K. Albrecht, M. Moeller, K. Nakagawa, S. Kitagawa and J. Groll, Nat. Chem., 2010, 2, 410–416 CrossRef CAS PubMed
.
- P. X. Gao, Y. Ding and Z. L. Wang, Nano Lett., 2003, 3, 1315–1320 CrossRef CAS
.
- K. Keis, L. Vayssieres, S.-E. Lindquist and A. Hagfeldt, Nanostruct. Mater., 1999, 12, 487–490 CrossRef
.
- Y. Dai, Y. Zhang, Q. K. Li and C. W. Nan, Chem. Phys. Lett., 2002, 358, 83–86 CrossRef CAS
.
- M.-C. Jeong, B.-Y. Oh, W. Lee and J.-M. Myoung, Appl. Phys. Lett., 2005, 86, 103105 CrossRef
.
- M. Bagheri, N. F. Hamedani, A. R. Mahjoub, A. A. Khodadadi and Y. Mortazavi, Sens. Actuators, B, 2014, 191, 283–290 CrossRef CAS
.
- M. Bagheri, A. A. Khodadadi, A. R. Mahjoub and Y. Mortazavi, Sens. Actuators, B, 2013, 188, 45–52 CrossRef CAS
.
- K. T. Johnson, T. E. Gribb, E. M. Smoak and I. A. Banerjee, Chem. Commun., 2010, 46, 1757–1759 RSC
.
- H. T. Ng, J. Li, M. K. Smith, P. Nguyen, A. Cassell, J. Han and M. Meyyappan, Science, 2003, 300, 1249 CrossRef CAS PubMed
.
- M. Y. Masoomi and A. Morsali, Coord. Chem. Rev., 2012, 256, 2921–2943 CrossRef CAS
.
- D. M. Ciurtin, Y.-B. Dong, M. D. Smith, T. Barclay and H.-C. zur Loye, Inorg. Chem., 2001, 40, 2825–2834 CrossRef CAS PubMed
.
- T. M. McPhillips, S. E. McPhillips, H.-J. Chiu, A. E. Cohen, A. M. Deacon, P. J. Ellis, E. Garman, A. Gonzalez, N. K. Sauter, R. P. Phizackerley, S. M. Soltis and P. Kuhn, J. Synchrotron Radiat., 2002, 9, 401–406 CrossRef CAS PubMed
.
- W. Kabsch, J. Appl. Crystallogr., 1993, 26, 795–800 CrossRef CAS
.
- G. Sheldrick, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- L. J. Barbour, J. Supramol. Chem., 2001, 1, 189–191 CrossRef CAS
.
- Crystal data for TMU-4: C42.25H31.25N4.75O10.75Zn2, M = 908.21, monoclinic, space group P21/c, a = 12.344(3) Å, b = 26.323(5) Å, c = 15.663(3) Å, β = 97.56(3)°, V = 5045.2(18) Å3, Z = 4, crystal size (mm3): 0.12 × 0.08 × 0.01, T = 173(2) K, Dcalc. = 1.196 g cm−3, R1 = 0.0936, wR2 = 0.2706, 59
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 reflections measured, 11
356 reflections measured, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 unique (Rint = 0.0560), R1 = 0.1156, wR2 = 0.2866 (all data), GOF on F2 = 1.049, F(000) = 1856, μ = 1.004 mm−1, Dρmax = 1.171 e Å−3.
143 unique (Rint = 0.0560), R1 = 0.1156, wR2 = 0.2866 (all data), GOF on F2 = 1.049, F(000) = 1856, μ = 1.004 mm−1, Dρmax = 1.171 e Å−3. - A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS
.
- M. Y. Masoomi, K. C. Stylianou, A. Morsali, P. Retailleau and D. Maspoch, Cryst. Growth Des., 2014, 14, 2092–2096 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Other figures, full synthetic and analytical details. CCDC 996860. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra09186h |
| This journal is © The Royal Society of Chemistry 2014 |