A study using quantum chemical theory methods on the intrinsic fluorescence emission and the possible emission mechanisms of PAMAM
Yan Ji and
Ying Qian*
School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, China. E-mail: yingqian@seu.edu.cn; jiyan98@163.com
First published on 24th October 2014
Abstract
In recent decades, it has been found and determined that poly-amidoamine (PAMAM) can give fluorescence emission under certain conditions. PAMAMs possess amide, primary amine and tertiary amine groups, which are not typical fluorescence emission groups. The fluorescence emission centers and mechanisms of PAMAM were studied. In this report we used quantum chemical TDDFT methods to calculate the absorption and emission states of the chemical components of PAMAM, which including amide, amide resonance structure imidic acid, amine and ammonium groups. The theory calculations showed that the imidic acid and tertiary ammonium components can give emission. The mechanisms for the formation of the amide resonance structure imidic acid and tertiary ammonium were discussed. The calculation results show that the imidic acid and tertiary ammonium groups are responsible for the fluorescence emission of PAMAM, which might help to explain the intrinsic-fluorescence phenomenon.
Introduction
Poly-amidoamine (PAMAM) dendrimers were first synthesized by Tomalia in 1985.1 In recent years, fluorescence emission phenomena of PAMAM have been proven.2 PAMAM dendrimers have water dissolvable and inside holes,3 several modifiable chemical groups,4 good biocompatibility,5 the ability to host nanoparticles,6 and small molecule or drug delivery abilities,7 in addition to fluorescence properties, which will attract the interest of researchers. Some other dendrimers such as poly(amino esters),8b poly(propylether imine),8e poly(propylene-imine) (PPI) dendrimer and poly(ethyleneimine) (PEI) dendrimer9 were found to give fluorescence emission, owing to intrinsic-fluorescence emission phenomena.10 Many elements which influence the emission of PAMAM have been considered, such as exposure to air,9 pH,11 oxidation,12 the distances of the amine groups13 and some other influences such as size and shape.14 In recent years there have been some common interpretations, but no definite conclusions on intrinsic-fluorescence had been reached.15The PAMAM dendrimers possess primary amines, amides and tertiary amines. There are no typical fluorescence emission groups in PAMAM and thus its fluorescence emission center and mechanism have been studied by many research groups.8 In some situations the PAMAM produced the amide resonance structure imidic acid (HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).16 15N NMR, 1H NMR, 13C NMR, N–H 2D NMR and IR spectra have determined this amide resonance structure to comprise imidic acid and tertiary ammonium.16 In this paper, a new mechanism for the fluorescence emission phenomenon of PAMAM has been proposed. Quantum chemical calculations prove that this resonance structure, imidic acid, can give fluorescence emission. The amide/imidic acid resonance structure transformation mechanism was also discussed in order to explain the PAMAM fluorescence emission. The tertiary ammonium exits in the structure of PAMAM and also takes part in the fluorescence emission. The emission and absorption states of the chemical moieties in PAMAM were calculated in order to examine its fluorescence centers by quantum chemical theory methods, which might help to explain the intrinsic-fluorescence phenomenon.
N).16 15N NMR, 1H NMR, 13C NMR, N–H 2D NMR and IR spectra have determined this amide resonance structure to comprise imidic acid and tertiary ammonium.16 In this paper, a new mechanism for the fluorescence emission phenomenon of PAMAM has been proposed. Quantum chemical calculations prove that this resonance structure, imidic acid, can give fluorescence emission. The amide/imidic acid resonance structure transformation mechanism was also discussed in order to explain the PAMAM fluorescence emission. The tertiary ammonium exits in the structure of PAMAM and also takes part in the fluorescence emission. The emission and absorption states of the chemical moieties in PAMAM were calculated in order to examine its fluorescence centers by quantum chemical theory methods, which might help to explain the intrinsic-fluorescence phenomenon.
Results and discussions
1. Fluorescence of PAMAM
PAMAM (Fig. 1) gave weak fluorescence when synthesized initially. However, the PAMAM dendrimers exposed to air or with acid added were found to give fluorescence emission. The fluorescence emission phenomena of PAMAM have been proven by many research groups.7–15 Fig. 2 shows the fluorescence emission spectrum of PAMAM-G1 in a CH2Cl2 solution, which shows that PAMAM-G1 gave strong fluorescence. The photographs in Fig. 3 show that 1G, 2G, 3G and hyper-branch PAMAM gave blue-green color fluorescence under 365 nm UV light after exposure to air. Fig. 4 shows that PAMAM-G1 gave UV-vis absorption peaks at 229 nm and 286 nm. These results all show that the PAMAM dendrimers can give fluorescence emission under certain conditions. The fluorescence of PAMAM was termed intrinsic-fluorescence, and the PAMAM fluorescence centers and mechanism need to be determined. | ||
| Fig. 3 Photographs of PAMAM-G1, PAMAM-G2, PAMAM-G3 and PAMAM hyper-branch. Top: in visible light, bottom: under 365 nm UV light. | ||
Spectral data16 determined that the amide resonance structure imidic acid exists in PAMAM. This imidic acid moiety (Fig. 5 P-02), which has a rigid co-planar and p–π conjugated structure and contains C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds and a hydroxyl electronic donor group, might be the group responsible for the fluorescence emission. Imine C
N double bonds and a hydroxyl electronic donor group, might be the group responsible for the fluorescence emission. Imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds can afford fluorescence emission under certain conditions.17 The C
N double bonds can afford fluorescence emission under certain conditions.17 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in imidic acid is similar to the C
N bond in imidic acid is similar to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in imines, and therefore may give fluorescence emission. Amide, imidic acid,16 primary amine, primary ammonium, tertiary amine, tertiary ammonium and amidium groups are all present in PAMAM. Quantum chemical theory calculation methods were used to test the fluorescence emission properties of these structural units in PAMAM.
N bond in imines, and therefore may give fluorescence emission. Amide, imidic acid,16 primary amine, primary ammonium, tertiary amine, tertiary ammonium and amidium groups are all present in PAMAM. Quantum chemical theory calculation methods were used to test the fluorescence emission properties of these structural units in PAMAM.
 | ||
| Fig. 5 The amide (P-01), imidic acid (P-02), primary amine (P-03), primary ammonium (P-04), tertiary amine (P-05), tertiary ammonium (P-06) and amidium (P-07) moieties in PAMAM (ref. 16) used for theory calculations. | ||
2. Theory calculations
P-01 and P-02 (Fig. 5) are small components within the key groups of PAMAM, and studying these in isolation can make the quantum chemical calculations easy. P-01 is the amide component of PAMAM, which was isolated from the PAMAM chains. P-02 is the amide resonance structure imidic acid component of PAMAM. The quantum chemical calculation results for P-01 and P-02 are listed in Tables 1 and 2.| Electron transitiona | Energy (eV) | Calculated wavelength (nm) | Main transition configuration | Oscillator strength f |
|---|---|---|---|---|
| a S0 → S1 is the absorption transition of P-01 (singlet). | ||||
| S0 → S1 | 3.195 | 388.01 | HOMO → LUMO: 0.70656 | 0.0007 |
| S0 → S1 | 5.106 | 242.82 | HOMO−1 → LUMO: 0.68179 | 0.0797 |
| HOMO−3 → LUMO: 0.15224 | ||||
| S0 → S1 | 6.883 | 180.11 | HOMO → LUMO+1: −0.44445 | 0.0092 |
| HOMO−2 → LUMO: 0.28272 | ||||
| HOMO−3 → LUMO: 0.45577 | ||||
| S0 → S1 | 7.009 | 176.89 | HOMO → LUMO+1: −0.52148 | 0.0691 |
| HOMO−1 → LUMO+1: 0.24424 | ||||
| HOMO−2 → LUMO: −0.29636 | ||||
| HOMO−3 → LUMO: −0.25948 | ||||
| S0 → S1 | 7.560 | 164.00 | HOMO → LUMO+2: 0.11041 | 0.0809 |
| HOMO−2 → LUMO: −0.51492 | ||||
| HOMO−3 → LUMO: 0.41561 | ||||
| Electron transitiona | Energy (eV) | Calculated wavelength (nm) | Main transition configuration | Oscillator strength f |
|---|---|---|---|---|
| a S0 → S1 is the absorption transition of P-02; S1 → S0 is the emission transition of P-02 (singlet); S0 is the ground state and S1 is the excitation state. | ||||
| S0 → S1 | 0.663 | 1868.03 | HOMO → LUMO: 0.72495 | 0.0000 |
| S0 → S1 | 4.017 | 308.61 | HOMO → LUMO: 0.19054 | 0.0434 |
| HOMO−1 → LUMO: 0.67201 | ||||
| S0 → S1 | 4.912 | 252.39 | HOMO → LUMO+1: −0.67133 | 0.0334 |
| HOMO−1 → LUMO: 0.16858 | ||||
| HOMO−2 → LUMO: 0.12149 | ||||
| S0 → S1 | 5.693 | 217.76 | HOMO−2 → LUMO: −0.66739 | 0.0220 |
| HOMO−3 → LUMO: 0.15285 | ||||
| S0 → S1 | 6.425 | 192.96 | HOMO → LUMO+2: 0.68424 | 0.0405 |
| HOMO−3 → LUMO: −0.11371 | ||||
| S0 → S1 | 6.920 | 179.16 | HOMO−3 → LUMO: 0.13505 | 0.0110 |
| HOMO−1 → LUMO+1: 0.68841 | ||||
| S1 → S0 | 0.663 | 1868.03 | LUMO → HOMO: −0.16421 | 0.0000 |
| S1 → S0 | 4.017 | 308.61 | LUMO → HOMO − 1: −0.10986 | 0.0434 |
The transitions of the components in the gas phase under vacuum were set for the calculations. The calculation of the whole dendrimer PAMAM-G0 can be carried out, but the contribution of each group (Fig. 5) towards the fluorescence emission can not be easily analysed. The calculations for each component were performed without setting the solvent environment, as the effect of solvent on individual components can not equal the effect of solvent on whole dendrimers. Therefore the transitions in the gas phase under vacuum were set to calculate and consider the fluorescence contribution of each component without set solvent environments.
The absorption and emission spectra of P-01 and P-02 were calculated by a time-dependent density functional theory (TDDFT) B3LYP/6-31G method using the Gaussian 0918 software package. Up to now, TDDFT19 has been the best method for calculating excitation energies, frequency-dependent response properties, photoabsorption spectra, and for emission calculations. DFT methods require large computer resources, and the small components (Fig. 5) allow for efficient calculations. The calculation data are listed in Tables 1, 2 and 3. The calculation data for the P-01 amide only gave absorption spectra without giving emission spectra. The amide resonance structure imidic acid P-02 afforded emission at 308.61 nm. The traditional amide structure gave no fluorescence emission. The amide resonance structure imidic acid includes C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds and is the key fluorescence emission group, as determined from these calculation results.
N double bonds and is the key fluorescence emission group, as determined from these calculation results.
| Electron transitionb | Energy (eV) | Calculated wavelength (nm) | Main transition configuration | Oscillator strength f | |
|---|---|---|---|---|---|
| a The primary amine (P-03), primary ammonium (P-04), tertiary amine (P-05), tertiary ammonium (P-06) and amidium (P-07) components in PAMAM (structures in Fig. 5).b S1 → S0 is the emission transition of the components (singlet); S0 is the ground state, S1 is the excitation state. | |||||
| P-03 | S1 → S0 | 0.3934 | 3151.86 | LUMO → HOMO: 0.36520 | 0.0002 |
| S1 → S0 | 0.5777 | 2146.18 | LUMO → HOMO: 0.10708 | 0.0006 | |
| P-04 | S1 → S0 | 1.2726 | 974.23 | LUMO → HOMO: −0.37303 | 0.0099 |
| S1 → S0 | 0.7101 | 1746.11 | LUMO → HOMO: −0.26941 | 0.0016 | |
| P-05 | S1 → S0 | — | — | — | — |
| P-06 | S1 → S0 | 3.3368 | 371.56 | LUMO → HOMO: −0.12593 | 0.0150 |
| S1 → S0 | 6.4936 | 190.93 | LUMO → HOMO: −0.10599 | 0.1388 | |
| S1 → S0 | 1.5639 | 792.78 | LUMO → HOMO: −0.44768 | 0.0176 | |
| S1 → S0 | 1.4019 | 884.43 | LUMO → HOMO: −0.31141 | 0.0177 | |
| S1 → S0 | 1.4154 | 875.96 | LUMO → HOMO: 0.30283 | 0.0182 | |
| S0 → S1 | 3.3368 | 371.56 | HOMO → LUMO: 0.71123 | 0.0150 | |
| P-07 | S1 → S0 | 0.1259 | 9845.10 | LUMO → HOMO: 0.37008 | 0.0008 |
Table 3 lists the calculated emission spectral data for the primary amine (P-03), primary ammonium (P-04), tertiary amine (P-05), tertiary ammonium (P-06) and amidium (P-07) components in PAMAM (structure in Fig. 5). The primary amine and primary ammonium gave fluorescence emission and the emission wavelengths were all larger than 900 nm and in the infrared range. The amidium also gave emission in the infrared range. Based on the theory calculation data, the tertiary amine gave no emission. The tertiary ammonium gave emissions at 370 nm, 190 nm, 790 nm, 870 nm, 880 nm and 980 nm, which show that, based on the theory calculated results, the tertiary ammonium has strong fluorescence emission abilities.
Ref. 9 shows that the blue emission of an oxygen-doped tertiary amine (triethylamine) was a key contributor to the fluorescence of the poly(amido amine) dendrimer. Ref. 13 shows that the distances of the tertiary amines affect the fluorescence emission of PAMAM. It was demonstrated that the tertiary amines in the branching units of hyperbranched polymers are key for keeping high fluorescence efficiency.20 These findings all show that tertiary amine or tertiary ammonium groups have taken part in fluorescence emission. From the data in Table 3, it is observed that the tertiary ammonium component can give emission.
Fig. 6 shows the HOMO−1, HOMO, LUMO and LUMO+1 molecular orbitals of PAMAM-G1 for the imidic acid structure. The HOMO−1 shows that the electron density is primarily located at the center of the PAMAM-G1 structure. The HOMO shows that the ground state electron density is gathered at the center of the PAMAM-G1 structure, but is spread further to the four branches as compared with the HOMO−1 molecular orbital. The LUMO shows that the excited state electron density is gathered at the center of the PAMAM-G1 structure, mainly on the tertiary amine components but also spread to the imidic acid components. The LUMO+1 shows that the excited state electron density is gathered mainly on the imidic acid and tertiary amine parts of the PAMAM-G1. Comparing the HOMO to the LUMO, the molecular orbital for the ground state spreads from the tertiary amine centers to the imidic acid components, and the electron density for the excited state is mainly located on the imidic acid structures. It can be deduced from Fig. 6 and the data in Tables 1, 2 and 3 that the imidic acid and tertiary ammonium components are the fluorescence emission centers in PAMAM-G1.
3. Possible mechanisms
The three dimensional fluorescence excitation/emission spectra of PAMAM-G1 in a water solution are shown in Fig. 7. They show that the PAMAM-G1 has fluorescence emission in the ca. 400–600 nm range, and fluorescence emission peaks at about 450–500 nm. Fig. 7 highlights well the fluorescence phenomena of the PAMAM dendrimers. The fluorescence mechanism of PAMAM may be explained from the chemical groups’ mechanisms, which include amide/imidic acid transfer and amine protonation. | ||
| Fig. 7 The three dimensional fluorescence spectra of PAMAM-G1 in a water solution (1 × 10−3 mol L−1). | ||
The mechanism for the transformation of the amide to the amide resonance structure imidic acid is shown in Fig. 8. Low pH values can help the forming of the imidic acid resonance structure from the amide. High pH values can help the transformation of the imidic acid resonance structure to the amide. The ratios of amide/imidic acid can be determined by NMR integrals. However, the ratios are affected by many factors, such as pH, temperature, or solvent conditions. A pH related experiment for PAMAM shows that low pH values or the addition of acids can enhance the fluorescence emission of PAMAM.9 These mechanisms can explain why PAMAM gives strong fluorescence when adding acids, which is owing to the formation of the imidic acid structure. The PAMAM gave weak fluorescence when adding bases due to the imidic acid structure transforming to the amide.
Fig. 9 and 10 show the transformation mechanisms for the primary amine/ammonium, amide/amidium and tertiary amine/tertiary ammonium pairs. The theory calculation results listed in Table 3 show that the ammonium components can give emissions. The primary amine, primary ammonium and amidium groups gave emissions around the infrared range. The amide and tertiary amine groups gave no emissions. The tertiary ammonium group can give emissions in the visible light range. The ammonium groups were often formed at low pH, and PAMAM gave strong fluorescence emissions at low pH values. From Table 3 we can deduce that the tertiary ammonium groups are one fluorescence emission center. Thus, the fluorescence emission groups of PAMAM are the imidic acid component (transformed from the amide component) and the tertiary ammonium component, as can be explained by these mechanisms.
The calculated data in Tables 2 and 3 show that there might be fluorescence resonance energy transfer (FRET)21 among the chemical groups of PAMAM. FRET is a photophysical processes whereby individual chromophores communicate their electronic states, providing means for transferring excitations from a donor to an acceptor. FRET is sensitive to intra- and intermolecular distances of <10 nm. From Tables 2 and 3, groups’ emissions overlap with other groups’ absorptions. The distances R between groups were <10 nm, which obey the FRET conditions. It can therefore be deduced that FRET occurs in PAMAM (Fig. 11). For example, the imidic acid group shows absorption and emission at about 300 nm, and the P-06 tertiary ammonium shows emission and absorption at about 370 nm; the absorption and emission are close and have some overlap. There are three bonds between the imidic acid and the tertiary ammonium, which means that the distance R is smaller than 10 nm. Fig. 12 shows the calculated fluorescence emission wavelength positions. P-02, P-04 and P-06 have overlaps in their absorption/emission positions which obey the FRET rules (absorptions/emissions have overlaps and the distances between the groups are smaller than 10 nm). These findings show that the imidic acid, primary ammonium, and tertiary ammonium groups exhibit FRET phenomena in PAMAM, which assist its fluorescence emission.
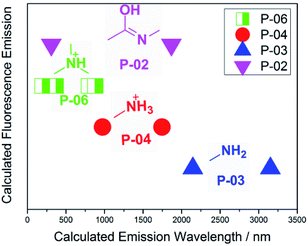 | ||
| Fig. 12 The calculated fluorescence emission wavelength positions of P-02, P-03, P-04 and P-06 (data from Tables 2 and 3). | ||
The key fluorescence emission groups in PAMAM are the imidic acid and tertiary ammonium groups, as indicated by the theory calculations. FRET may contribute to the fluorescence emission processes of PAMAM. The related mechanisms can partly explain the relationship between the fluorescence phenomena and the chemical structures of the PAMAM dendrimers.
Experimental methods
1. Synthesis
Syntheses of PAMAM 0.5G, 1.0G, 1.5G, 2.0G, 3.0G and hyper-branch:1Methyl acrylate (MA) and ethylenediamine (EDA) were used as substrates.
(1) The Michael-addition of amine groups in EDA to MA at 50 °C in a methanol solution afforded the dendritic product of 0.5 generation (G) with terminal ester groups.
(2) Amidation of the terminal ester groups of the 0.5G dendrimer by dissolution in a methanol solution with excess EDA at 50 °C afforded the 1G dendrimer with terminal amine groups.
(3) Distillation of the excess EDA under reduced pressure and washing with ethyl ether afforded the purified 1G dendrimer. The PAMAM dendrimers were found to be yellow sticky liquids.
(4) Repetition of steps (1), (2) and (3) afforded 1.5G, 2.0G and 3.0G.
(5) Equal amounts of MA and EDA were added into methanol and the solution was stirred at 50 °C for 24 h. After distillation of methanol, hyper-branch PAMAM was obtained.
Conclusions
The spectral data show that PAMAM dendrimers exhibit fluorescence emission phenomena. The amide of PAMAM exists in its resonance form, imidic acid. The tertiary amine transforms into ammonium under certain conditions. Characterizations confirmed that imidic acid and tertiary ammonium groups exist in PAMAM. Quantum chemical theory calculations show that the imidic acid and tertiary ammonium components can give fluorescence emission. The amide/imidic acid transformation mechanism and the tertiary amine/ammonium protonation mechanism were discussed and can explain the high fluorescence emission phenomena of PAMAM in low pH or after the addition of acid. It can be concluded that the imidic acid and tertiary ammonium components co-contribute to the fluorescence emission of PAMAM. These calculation results can help to explain and understand the intrinsic-fluorescence phenomena of dendrimers.Acknowledgements
Financial support from the National Natural Science Foundation of China (no.61178057) and the Scientific Research Foundation of Graduate School of Southeast University (no.YBPY1209) is greatly appreciated.Notes and references
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117–132 CrossRef CAS.
- (a) K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45(28), 4562–4588 CrossRef CAS PubMed; (b) A. M. Caminade, A. Hameau and J. P. Majoral, Chem.–Eur. J., 2009, 15(37), 9270–9285 CrossRef CAS PubMed; (c) G. R. Newkome and C. D. Shreiner, Polymer, 2008, 49(1), 1–173 CrossRef CAS PubMed; (d) U. Boas, J. B. Christensen and P. M. H. Heegaard, J. Mater. Chem., 2006, 16(38), 3786–3798 RSC; (e) C. L. Larson and S. A. Tucker, Appl. Spectrosc., 2001, 55, 679–683 CrossRef CAS.
- J. F. Jansen, E. M. de Brabander-van den Berg and E. W. Meijer, Science, 1994, 266, 1226–1229 CAS.
- (a) A. I. Cooper, J. D. Londono, G. Wignall, J. B. McClain, E. T. Samulski, J. S. Lin, A. Dobrynin, M. Rubinstein, A. L. C. Burke, J. M. J. Frechet and J. M. DeSimone, Nature, 1997, 389, 368–371 CrossRef CAS PubMed; (b) R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS PubMed; (c) Y. Ji and Y. Qian, RSC Adv., 2014, 4, 25510–25519 RSC.
- (a) C. S. Braun, M. T. Fisher, D. A. Tomalia, G. S. Koe, J. G. Koe and C. R. Middaugh, Biophys. J., 2005, 88(6), 4146–4158 CrossRef CAS PubMed; (b) X. Y. Shi, I. J. Majoros, A. K. Patri, X. D. Bi, M. T. Islam, A. Desai, T. R. Ganser and J. R. Baker Jr, Analyst, 2006, 131(3), 374–381 RSC; (c) L. Fernandez, M. Gonzalez, H. Cerecetto, M. Santoc and J. Silberet, J. Supramol. Chem., 2006, 18(8), 633–643 CrossRef CAS; (d) K. J. Landmark, S. DiMaggio, J. Ward, C. Kelly, S. Vogt, S. Hong, A. Kotlyar, A. Myc, T. P. Thomas, J. E. Penner-Hahn, J. R. Baker Jr, M. M. B. Holl and B. G. Orr, ACS Nano, 2008, 2, 773–783 CrossRef CAS PubMed; (e) R. K. Tekade, P. V. Kumar and N. K. Jain, Chem. Rev., 2009, 109, 49–87 CrossRef CAS PubMed.
- (a) S. P. Mukherjee and H. J. Byrne, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9(2), 202–211 CrossRef CAS PubMed; (b) C. M. Lamy, O. Sallin, C. Loussert and J. Y. Chatton, ACS Nano, 2012, 6(2), 1176–1187 CrossRef CAS PubMed; (c) Y. J. Tsai, C. C. Hu, C. C. Chu and T. Imae, Biomacromolecules, 2011, 12(12), 4283–4290 CrossRef CAS PubMed; (d) M. F. Ottaviani, F. Montalti, N. J. Turro and D. A. Tomalia, J. Phys. Chem. B, 1997, 101, 158–166 CrossRef CAS.
- (a) S. Sadekar and H. Ghandehari, Adv. Drug Delivery Rev., 2012, 64(6), 571–588 CrossRef CAS PubMed; (b) L. Albertazzi, B. Storti, L. Marchetti and F. Beltram, J. Am. Chem. Soc., 2010, 132(51), 18158–18167 CrossRef CAS PubMed; (c) B. K. Biswal, M. Kavitha, R. S. Verma and E. Prasad, Cytotechnology, 2009, 61(1–2), 17–24 CrossRef PubMed; (d) H. Wang, H. B. Shi and S. K. Yin, Exp. Ther. Med., 2011, 2, 777–781 CAS; (e) F. Zeng and S. C. Zimmerman, Chem. Rev., 1997, 97, 1681–1712 CrossRef CAS PubMed; (f) A. W. Bosman, H. M. Janssen and E. W. Meijer, Chem. Rev., 1999, 99, 1665–1966 CrossRef CAS PubMed; (g) L. J. Twyman, A. S. H. King and I. K. Martin, Chem. Soc. Rev., 2002, 31, 69–82 RSC.
- (a) D. J. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204–13205 CrossRef CAS PubMed; (b) D. C. Wu, Y. Liu, C. B. He and S. H. Goh, Macromolecules, 2005, 38(24), 9906–9909 CrossRef CAS; (c) Y. F. Fan, Y. G. Fan and Y. N. Wang, J. Appl. Polym. Sci., 2007, 106(3), 1640–1647 CrossRef CAS; (d) P. L. Wang, X. Wang, K. Meng, S. Hong, X. Liu, H. Cheng and C. C. Han, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(10), 3424–3428 CrossRef CAS; (e) G. Jayamurugan, C. P. Umesh and N. Jayaraman, Org. Lett., 2008, 10, 9–12 CrossRef CAS PubMed.
- C. C. Chu and T. Imae, Macromol. Rapid Commun., 2009, 30(2), 89–93 CrossRef CAS PubMed.
- (a) R. A. Beecroft, R. S. Davidson and T. D. Whelan, J. Chem. Soc., Perkin Trans. 2, 1985, 2, 1069–1072 RSC; (b) C. Freeman, M. McEwan, R. Claridge and L. Phillips, Chem. Phys. Lett., 1971, 8, 77–78 CrossRef CAS.
- (a) G. Saravanan and H. Abe, J. Photochem. Photobiol., A, 2011, 224(1), 102–109 CrossRef CAS PubMed; (b) D. J. Wang, T. Imae and M. Miki, J. Colloid Interface Sci., 2007, 306(2), 222–227 CrossRef CAS PubMed.
- W. I. Lee, Y. Bae and A. J. Bard, J. Am. Chem. Soc., 2004, 126, 8358–8359 CrossRef CAS PubMed.
- L. Cao, D. D. Jia, S. F. Wang, Y. L. Rong, C. Liu and D. J. Wang, Chem. Lett., 2014, 43(2), 246–248 CrossRef CAS.
- W. Yang and C. Y. Pan, Macromol. Rapid Commun., 2009, 30(24), 2096–2101 CrossRef CAS PubMed.
- (a) M. J. Chen and M. Z. Yin, Prog. Polym. Sci., 2014, 39, 365–395 CrossRef CAS PubMed; (b) D. J. Wang, D. D. Jia, X. F. Zheng, L. Liu, H. Y. Tian and S. F. Wang, Gaofenzi Tongbao, 2011, 5, 27–33 Search PubMed; (c) S. H. Niu, Y. Wang, D. G. Fu and Y. M. Sun, Gaofenzi Tongbao, 2008, 2, 59–65 Search PubMed.
- Y. Ji, X. L. Yang and Y. Qian, RSC Adv., 2014, 4, 49535–49540 RSC.
- (a) J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC; (b) J. S. Wu, W. M. Liu, X. Q. Zhuang, F. Wang, P. F. Wang, S. L. Tao, X. H. Zhang, S. K. Wu and S. T. Lee, Org. Lett., 2007, 9, 33–36 CrossRef CAS PubMed; (c) W. Liu, L. Xu, R. Sheng, P. Wang, H. Li and S. Wu, Org. Lett., 2007, 9, 3829–3832 CrossRef CAS PubMed; (d) D. Ray and P. K. Bharadwaj, Inorg. Chem., 2008, 47, 2252–2254 CrossRef CAS PubMed; (e) V. Chandrasekhar, P. Bag and M. D. Pandey, Tetrahedron, 2009, 65, 9876–9883 CrossRef CAS PubMed; (f) H. S. Jung, K. C. Ko, J. H. Lee, S. H. Kim, S. Bhuniya, J. Y. Lee, Y. Kim, S. J. Kim and J. S. Kim, Inorg. Chem., 2010, 49, 8552–8557 CrossRef CAS PubMed; (g) M. Suresh, A. K. Mandal, S. Saha, E. Suresh, A. Mandoli, R. D. Liddo, P. P. Parnigotto and A. Das, Org. Lett., 2010, 12, 5406–5409 CrossRef CAS PubMed; (h) Z. Li, M. Yu, L. Zhang, M. Yu, J. Liu, L. Wei and H. Zhang, Chem. Commun., 2010, 46, 7169–7171 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) E. Runge and E. K. U. Gross, Phys. Rev. Lett., 1984, 52(12), 997 CrossRef CAS; (b) Time-Dependent Density Functional Theory, ed. M. A. L. Marques, C.A. Ullrich, F. Nogueira, A. Rubio, K. Burke and E.K.U. Gross, Springer-Verlag, 2006 Search PubMed.
- M. Sun, C. Y. Hong and C. Y. Pan, J. Am. Chem. Soc., 2012, 134, 20581–20584 CrossRef CAS PubMed.
- L. Stryer and R. P. Haugland, Proc. Natl. Acad. Sci. U. S. A., 1967, 58, 719 CrossRef CAS; B. W. Van der Meer, G. Coker III and S. Y. S. Chen, Resonance energy transfer theory and data, VCH, New York, 1994 Search PubMed; J. R. Lakowicz, Principles of fluorescence spectroscopy, Kluwer Academic/Plenum Publishers, New York, 2nd edn, 1999 Search PubMed; M. Merkx, Rational design of FRET-based sensor proteins, Reviews in Fluorescence, 2008, pp. 69–87 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |








