Manganese(III)meso-tetrakis(4-N-methylpyridyl)-porphyrin and mediator thionine co-decorated DNA nanowires for sensitive electrochemical monitoring of mercury(II)
G. P. Liua,
Y. M. Wub,
Y. L. Yuanab,
Y. Q. Chai*b,
S. Q. Wei*a and
D. J. Zhanga
aCollege of Resources and Environments, Southwest University, Chongqing 400715, PR China. E-mail: sqwei@swu.edu.cn
bKey Laboratory of Luminescent and Real-Time Analytical Chemistry, Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, PR China. E-mail: yqchai@swu.edu.cn
First published on 19th September 2014
Abstract
We developed a novel electrochemical DNA biosensor for mercury(II) ion (Hg2+) detection on the basis of manganese(III) meso-tetrakis(4-N-methylpyridyl)-porphyrin (MnTMPyP) and electron mediator thionine (Thi) co-decorated DNA nanowires for signal amplification. The T-rich capture DNA assembled on the electrode could successfully immobilize the primer DNA via specific base-pairing, which triggered the hybridization chain reaction (HCR) to form long DNA nanowires with the aim of loading abundant MnTMPyP and electron mediator Thi. In the electrolyte containing H2O2, the MnTMPyP loaded in the DNA nanowires showed superior peroxidase-like activity and electrocatalyzed the reduction of H2O2, promoting the redox reaction of Thi with a dramatically amplified electrochemical signal. However, in the presence of target Hg2+, Hg2+-mediated thymine base pairs (T–Hg2+–T) are formed between the two neighboring T-rich capture DNAs, which resulted in the release of the MnTMPyP and Thi co-decorated DNA nanowires from the electrode surface, providing a reduced readout signal for the quantitative electrochemical detection of Hg2+. The results showed that the proposed electrochemical DNA biosensor was highly sensitive to Hg2+ in the concentration of 1.0 ng L−1 to 107 ng L−1 with a detection limit of 0.5 ng L−1 (2.5 pM), and it also exhibited excellent selectivity against other interferential metal ions.
Introduction
Contamination with metal ions has created a pressing public health concern in living systems. They can induce toxic effects in animals and plants when circulating in groundwater and soil, and further delay physical or mental development in the human body, causing damage to the nervous, renal, immune, and cardiovascular systems once introduced into the body excessively.1 Therefore, sensitive and on-site tracking of toxic metal ions is highly desirable in the environmental and food industries. Recent researches indicate that metal ions, such as Ag+, Hg2+, Cu2+, Pb2+, and Ni2+, could bind to specific nucleic acid bases to form metal ion-mediated base pairs, in which the hydrogen bonds of Watson–Crick (W–C)-type base pairs are replaced by metal base bonds.2 This novel interaction of nucleic acids with metal ions shows a promising prospect of DNA-based biosensors in the detection of metal ions. Accordingly, various strategies including fluorescence,3 electrochemistry (EC),4 electrochemiluminescence5 and colorimetry6 have been employed for the sensitive detection of metal ions. Among them, EC-based biosensors have attracted significant interest because of their inherent simplicity, fast response, low cost and high sensitivity.Manganese(III) meso-tetrakis (4-N-methylpyridyl)-porphyrin (MnTMPyP) is a typical manganese porphyrin that contains manganese as the central metal atom.7 It can interact with both the AT and GC base pairs of double-strand DNA (dsDNA) by groove binding and exhibit superior peroxidase-like activity.8 Compared with the commonly used hemin/G-quadruplex, a complex with iron porphyrin hemin functions as a G-rich, single-strand DNA and shows peroxidase-like activity; the MnTMPyP–dsDNA does not require a specific sequence, and the amount of MnTMPyP loaded in the dsDNA scaffold is relatively much larger, which provides a new avenue for the preparation of simple and sensitive electrochemical DNA biosensors with high catalytic efficiency. However, to the best of our knowledge, only a few relevant works have been reported.
Herein, based on the MnTMPyP and electron mediator thionine (Thi) co-decorated DNA nanowires, we prepared a simple and sensitive electrochemical DNA biosensor for the trace detection of toxic mercury(II) ion (Hg2+), which may cause DNA damage, brain damage, organic functions disablement, and immune system homeostasis disruption.9 The T-rich capture DNA was first assembled on the nano-Au-modified glass carbon electrode (GCE). Through specific base-pairing, the primer DNA was thus modified on the electrode surface to trigger the hybridization chain reaction (HCR) with the aim of forming the MnTMPyP and Thi co-decorated DNA nanowires. In the electrolyte containing H2O2, the MnTMPyP loaded in the DNA nanowires showed superior peroxidase-like activity, which quickly electrocatalyzed the reduction of H2O2, promoting the redox reaction of Thi with a dramatically amplified electrochemical signal. However, in the presence of target Hg2+, Hg2+-mediated thymine base pairs (T–Hg2+–T) were formed between the two neighboring T-rich capture DNA,10 which resulted in the release of MnTMPyP and Thi co-decorated DNA nanowires from electrode surface, providing a reduced readout signal for the quantitative electrochemical detection of Hg2+. The proposed biosensor exhibited excellent sensitivity with a detection limit of 2.5 pM and also had high selectivity toward Hg2+. Furthermore, the practical applications of the DNA biosensor were investigated by detecting spiked Hg2+ in tap water, lake water, and river water samples.
Experimental
Chemicals and materials
Manganese(III) meso-tetrakis(4-N-methylpyridyl)-porphyrin (MnTMPyP), thionine (Thi), gold chloride (HAuCl4) and hexanethiol (96%, HT) were obtained from Sigma-Aldrich (St. Louis, Mo., USA). Tris-hydroxy-methylaminomethane hydrochloride (Tris) was obtained from Roche (Switzerland). K3[Fe(CN)6] and K4[Fe(CN)6] were obtained from Beijing Chemical Reagent Co. (Beijing, China). The referenced sequences were synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China).DNA stock solutions were obtained by dissolving DNA in 20 mM Tris–HCl buffer (pH 7.4) containing 140 mM NaCl, 5 mM KCl and 1 mM MgCl2. Unless otherwise mentioned, all other reagents were of analytical grade and were used without further purification or treatment. Double-distilled water was used throughout this study.
Electrochemical measurements
Cyclic voltammetry (CV) and differential pulse voltammograms (DPV) were performed on a CHI 660D electrochemical workstation (Shanghai Chenhua Instrument, China) using a conventional three-electrode system with a modified glassy carbon electrode as the working electrode (GCE, Φ = 4 mm), a platinum wire as the auxiliary electrode, and a saturated calomel electrode (SCE) as the reference electrode. The electrodeposition of gold nanoparticles (nano-Au) on the electrode surface was carried out in an aqueous solution of 1% AuCl4− (ω%) at a constant potential of −0.2 V for 30 s.The fabrication of the DNA biosensor
Details of the DNA biosensor fabrication are schematized in Scheme 1. The GCE was first polished sequentially with 1.0 and 0.3 μm alumina slurry, followed by ultrasonic cleaning in ethanol and double-distilled water for 5 min each. In order to assemble the capture DNA, the cleaned GCE was then soaked in an HAuCl4 aqueous solution and electrodeposited at −0.2 V for 30 s to form a gold nanoparticle (nano-Au) layer. After dropping 20 μL capture DNA solution (1 μM) on the nano-Au/GCE surface and incubating for 16 h at room temperature, 1 mM HT was used for blocking the remaining active sites on the electrode surface. Subsequently, the modified HT/capture DNA/nano-Au/GCE was subjected to incubation with 20 μL primer DNA solution for 2 h at 37 °C (1 μM). To form the long DNA nanowires with abundant MnTMPyP and electron mediator Thi on the electrode surface, a 30 μL mixture containing 1 μM H1, 1 μM H2, 0.3 mM electron mediator Thi and 0.15 mM MnTMPyP was then coated onto the obtained primer DNA/HT/capture DNA/nano-Au/GCE and incubated for 2 h at 37 °C. The resultant DNA biosensor was stored at 4 °C until use.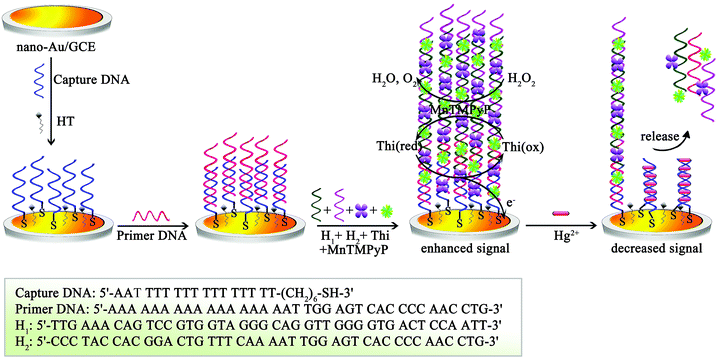 | ||
| Scheme 1 Schematic illustration of the MnTMPyP and mediator thionine co-decorated DNA nanowires for sensitive mercury(II) ion electrochemical DNA biosensors. | ||
Electrochemical detection of Hg2+
Various concentrations of Hg2+ were eventually added to the resultant DNA biosensor and incubated for 30 min. A controlled reaction without Hg2+ was set up in parallel. Finally, the resultant DNA biosensor was investigated by differential pulse voltammetry (DPV) from 0 to −500 mV (vs. SCE) in the PBS (pH 7.0) containing 0.36 mM H2O2. According to the relationship between the Hg2+ concentration and the decreased current response, the quantitative electrochemical detection of Hg2+ could be realized.Results and discussion
Electrochemical characteristics of the proposed DNA biosensor
Cyclic voltammetry (CV) was used for studying the fabricating process of the proposed DNA biosensor. As shown in Fig. 1A, the bare GCE displayed a well-defined redox peak in 0.1 M PBS containing 5.0 mM [Fe(CN)6]3−/4− (pH 7.0) (curve a). The peak current increased obviously after the electrodeposition of a conductive nano-Au layer (curve b). However, after the modification of capture DNA, the electrode exhibited a decreased peak current (curve c). This is quite reasonable because the immobilization of capture DNA on nano-Au/GCE resulted in a highly negatively charged surface, reducing the electron-transfer rate. Moreover, the peak current kept decreasing after the consecutive assembly of inset HT and primer DNA (curve d and e, respectively).Because the long DNA nanowires were formed on the electrode containing abundant MnTMPyP and electron mediator Thi, we employed differential pulse voltammograms (DPV) to verify the assembly of DNA nanowires. Curve a in Fig. 1B was the obtained primer DNA/HT/capture DNA/nano-Au/GCE investigated in the electrolyte of 0.1 M PBS. As expected, no obvious peak current was obtained. However, after the assembly of DNA nanowires with abundant MnTMPyP and electron mediator Thi, a distinct peak current could be obtained (curve b in Fig. 1B), suggesting the successful assembly of DNA nanowires on electrode. In addition, we investigated the electrocatalysis characteristics of MnTMPyP in the sensitivity improvement of the proposed DNA biosensor. The primer DNA/HT/capture DNA/nano-Au/GCE with the assembly of the DNA nanowires was subjected to investigation in 0.1 M PBS containing 0.36 mM H2O2 (curve c in Fig. 1B). As seen from curve c in Fig. 1B, the peak current obviously increased compared with that obtained only in PBS (curve b in Fig. 1B), which gave conclusive evidence of the electrocatalysis characteristics of MnTMPyP in the sensitivity improvement of the DNA biosensor.
Moreover, the Raman spectra were used to verify that Hg2+ cannot exchange with Mn3+ chelated in porphyrin. We investigated the solutions of pure MnTMPyP, the DNA nanowires with MnTMPyP and Thi, and the DNA nanowires with MnTMPyP and Thi after the addition of Hg2+. As shown in Fig. 2, curve a displayed the typical spectra of porphyrin.11 However, after MnTMPyP and Thi assembled on the formed DNA nanowires, the spectra became relatively smooth and the primary peaks for MnTMPyP shifted slightly (curve b). In addition, there were no obvious changes compared with that of curve b after the addition of Hg2+ into DNA nanowires + MnTMPyP + Thi (curve c), suggesting that Hg2+ cannot exchange with Mn3+ that chelated in porphyrin in our work.
 | ||
| Fig. 2 Raman spectra of (a) MnTMPyP, (b) DNA nanowires + MnTMPyP + Thi and (c) DNA nanowires + MnTMPyP + Thi after adding Hg2+. | ||
The amplification property of MnTMPyP
To verify the amplification property of MnTMPyP, the proposed DNA biosensor before and after target incubation was investigated in PBS with and without H2O2, respectively. As shown in Fig. 3A, the electrochemical signal of the proposed DNA biosensor investigated in PBS changed 3.09 μA before (curve a) and after (curve b) target incubation. However, the electrochemical signal displayed a significantly larger change (7.54 μA, Fig. 3B) when the proposed DNA biosensor was investigated in PBS containing H2O2 before (curve a) and after (curve b) target incubation, suggesting a superior amplification property of MnTMPyP.Optimization of experimental conditions
Generally, it takes some time to form the long DNA nanowires with abundant MnTMPyP and electron mediator Thi. To achieve an optimal electrochemical signal, the effect of incubation time between H1 and H2 on the electrochemical signal of the DNA sensor was investigated. 30 μL mixture containing 1 μM H1, 1 μM H2, 0.3 mM electron mediator Thi and 0.15 mM MnTMPyP was coated on the obtained primer DNA/HT/capture DNA/nano-Au/GCE and incubated for various periods at 37 °C. As indicated in Fig. 4A, the electrochemical signal obtained for 0.1 M PBS increased with increasing incubation time and reached a peak after 2 h. Therefore, an incubation time of 2 h was selected for the sensitive detection of Hg2+ at an acceptable throughput.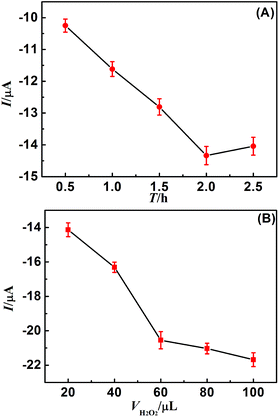 | ||
| Fig. 4 (A) Optimization of DNA nanowire reaction time and (B) H2O2 concentration in the electrolyte. | ||
H2O2 concentration is a key factor influencing the electrocatalysis efficiency of MnTMPyP in the sensitivity improvement of the proposed DNA biosensor. To investigate the effect of H2O2 concentration on the electrocatalysis efficiency of MnTMPyP, the proposed DNA biosensor with MnTMPyP and electron mediator Thi was subjected to characterization in the electrolyte of 1 mL 0.1 M PBS containing various H2O2 concentration. As shown in Fig. 4B, the electrochemical signal increased with increasing H2O2 concentration, and the increasing tendency of the electrochemical signal becomes very slow after the addition of 60 μL 6.00 mM H2O2 (equivalent to 0.36 mM H2O2 in electrolyte). Therefore, the optimal concentration of H2O2 for the electrocatalytic system was selected to be 0.36 mM.
Electrochemical detection of Hg2+ with the proposed DNA biosensor
The sensitivity and dynamic range of the as-prepared electrochemical DNA biosensors were evaluated with Hg2+ standards based on the amplification of HCR and MnTMPyP. A differential pulse voltammogram (DPV) measurement was carried out in 1 mL 0.1 M PBS containing 0.36 mM H2O2. Fig. 5 showed the relationship between the peak current and the concentration logarithm of Hg2+. As expected, the peak current decreased with the increasing concentration of Hg2+ and showed a good linear relationship in the range of 1.0 ng L−1 to 107 ng L−1 for Hg2+. The linear equation was I = 18.26 − 1.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cHg2+, where I is the peak current value and c is the concentration of Hg2+, and the correlation coefficient was 0.9936. With the amplification of HCR and MnTMPyP, the proposed DNA biosensor showed high sensitivity with a detection limit of 0.5 ng L−1 (2.5 pM), which was lower than the toxic level for Hg2+ defined by the U.S. Environmental Protection Agency in potable water (<10 nM). Thus, the developed DNA biosensor fully met the requirement of Hg2+ analysis. Additionally, the proposed DNA biosensor was compared with that of the reported sensors, and the results are listed in Table 1. From Table 1, we can see that the proposed biosensor showed a relatively lower detection limit and a wider linear range, suggesting a superior property of the proposed biosensor.
cHg2+, where I is the peak current value and c is the concentration of Hg2+, and the correlation coefficient was 0.9936. With the amplification of HCR and MnTMPyP, the proposed DNA biosensor showed high sensitivity with a detection limit of 0.5 ng L−1 (2.5 pM), which was lower than the toxic level for Hg2+ defined by the U.S. Environmental Protection Agency in potable water (<10 nM). Thus, the developed DNA biosensor fully met the requirement of Hg2+ analysis. Additionally, the proposed DNA biosensor was compared with that of the reported sensors, and the results are listed in Table 1. From Table 1, we can see that the proposed biosensor showed a relatively lower detection limit and a wider linear range, suggesting a superior property of the proposed biosensor.
 | ||
| Fig. 5 Corresponding calibration curve toward Hg2+ with various concentrations in the electrolyte of 0.1 M PBS containing 0.36 mM H2O2. | ||
| Analytical method | Detection limit | Linear range | Ref. |
|---|---|---|---|
| Electrochemiluminescence | 0.105 nM | 0.2–20 nM | 12 |
| Field-effect transistors | 1.2 nM | — | 13 |
| Differential pulse voltammograms | 0.01 nM | 0.05–350 nM | 14 |
| Differential pulse voltammograms | 2.5 nM | 5.0 to 106 nM | 15 |
| Chemiluminescent | 21 nM | 50–100 nM | 16 |
| Surface-enhanced Raman scattering | 0.34 nM | 1.0–100 nM | 17 |
| Fluorescence | 3 nM | 10–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM 000 nM |
18 |
| Differential pulse voltammograms | 0.5 ng L−1 (2.5 pM) | 1 to 107 ng L−1 (5 pM to 5 × 107 nM) | Our work |
Selectivity and reproducibility of the proposed DNA biosensor
The selectivity of the electrochemical DNA biosensor was evaluated by testing it against other metal ions: As3+, Ag+ and Pb2+. Significantly higher current change was observed with the target Hg2+ than those with other metal ions (Fig. 6). The results clearly revealed the high selectivity of the developed electrochemical DNA biosensor toward Hg2+. Additionally, the proposed DNA biosensor after the incubation of the mixture containing As3+, Ag+, Pb2+, and Hg2+ was also investigated. In addition, the current change was close to that of Hg2+ only, which further verified the high selectivity of the developed electrochemical DNA biosensor toward Hg2+. The reproducibility of the proposed DNA biosensor was investigated by the intra- and inter-assays. The intra-assay was performed by analyzing five times using one proposed biosensor with the incubation of 1 ng L−1 Hg2+, and the inter-assay was performed by determining 1 ng L−1 Hg2+ using five different proposed biosensors. Experimental results revealed that the coefficients of the variation (CVs) of the intra- and inter-assays were not more than 5%, suggesting the acceptable reproducibility of the proposed DNA biosensor. | ||
| Fig. 6 Specificity and interfering effects of the sample matrix components on the electrochemical signal of the proposed biosensor. | ||
Analysis of tap water, lake water, and river water samples
In order to further explore the potential application of the proposed electrochemical DNA biosensors in practical samples, recovery experiments were performed by using standard addition methods in tap water, lake water, and river water. The sample was directly spiked with certain amounts of Hg2+ standard solution. As shown in Table 2, the proposed electrochemical DNA biosensor exhibited good consistencies between the actual and the measured Hg2+ concentrations, which is comparable to conventional Hg2+ detection methods (AFS), indicating a promising tool for Hg2+ detection with sufficient precision and accuracy, even in real tap water, lake water, and river water sample matrices.| Samples | Added | Foundb | Recovery (%) | AFSc | Relative errord (%) |
|---|---|---|---|---|---|
| a The DPV assays were carried out within the range of −0.5–0.0 V in the electrolyte of 0.1 M PBS containing 0.36 mM H2O2. Lake water was obtained from Chongdehu of Southwest University, China; the river water was obtained from the Yangtze River, China.b Each sample was analyzed using proposed biosensors, and all values were obtained as the average of the three repetitive determinations ± standard deviation (mean ± SD).c The concentration of Hg2+ in the water samples was certified using AFS.d Proposed biosensor vs. AFS method. | |||||
| Tap water 1 | 10 | 9.4 ± 0.6 | 93.8 | 9.8 ± 0.4 | 4.3 |
| Tap water 2 | 1000 | 1083.9 ± 67.2 | 108.4 | 1018.0 ± 43.1 | −6.1 |
| Lake water 1 | 10 | 10.7 ± 0.5 | 107.2 | 10.5 ± 0.3 | −1.9 |
| Lake water 2 | 1000 | 910.6 ± 46.4 | 91.1 | 980.6 ± 36.2 | 7.7 |
| River water 1 | 10 | 9.1 ± 0.6 | 91.3 | 9.6 ± 0.5 | 5.5 |
| River water 2 | 1000 | 906.5 ± 49.9 | 90.7 | 973.8 ± 28.3 | 7.4 |
Conclusions
We have successfully fabricated a simple and sensitive electrochemical DNA biosensor for the trace detection of Hg2+ based on the amplification of MnTMPyP and electron mediator Thi co-decorated DNA nanowires. The MnTMPyP interacted with both AT and GC base pairs of dsDNA by groove binding without the need for a specific sequence. This made the fabrication process of the proposed biosensor much simpler and largely improved the immobilization amount of the MnTMPyP, compared with the tradition hemin/G-quadruplex. More importantly, the MnTMPyP exhibited superior peroxidase-like activity, which electrocatalyzed the reduction of the H2O2, promoting the redox reaction of Thi with a dramatically amplified electrochemical signal. Additionally, the formed DNA nanowires could also effectively improve the immobilization amount of MnTMPyP and electron mediator Thi, further improving the sensitivity of the proposed biosensor. Considering the high sensitivity and selectivity of this electrochemical Hg2+ biosensor, it is expected to hold significant promise in the environmental monitoring of mercury.Acknowledgements
The authors are grateful to the National Basic Research Program of China (973 Program) (2013CB430003-04), the National Natural Science Foundation of China (21075100, 21275119, 40971147), the China Postdoctoral Science Foundation (2014M550454), the Chongqing Postdoctoral Science Foundation (Xm2014022) and the Fundamental Research Funds for the Central Universities (XDJK2014C138).Notes and references
- Y. H. Luo, L. L. Xu, A. H. Liang, A. P. Deng and Z. L. Jiang, RSC Adv., 2014, 4, 19234 RSC.
- (a) J. Kondo, T. Yamada, C. Hirose, I. Okamoto, Y. Tanaka and A. Ono, Angew. Chem., 2014, 126, 2417 CrossRef; (b) Y. Zheng, C. Yang, F. Yang and X. R. Yang, Anal. Chem., 2014, 86, 3849 CrossRef CAS PubMed; (c) J. Liu and Y. Lu, J. Am. Chem. Soc., 2005, 127, 12677 CrossRef CAS PubMed; (d) Y. Xiao, A. A. Rowe and K. W. Plaxco, J. Am. Chem. Soc., 2007, 129, 262 CrossRef CAS PubMed; (e) D. Mazumdar, J. Liu, G. Lu, J. Zhou and Y. Lu, Chem. Commun., 2010, 46, 1416 RSC.
- (a) A. Porchetta, A. Vallee-Belisle, K. W. Plaxco and F. Ricci, J. Am. Chem. Soc., 2013, 135, 13238 CrossRef CAS PubMed; (b) D. Huang, C. Niu, X. Wang, X. Lv and G. Zeng, Anal. Chem., 2013, 85, 1164 CrossRef CAS PubMed.
- (a) Z. Z. Lin, X. H. Li and H. B. Kraatz, Anal. Chem., 2011, 83, 6896 CrossRef CAS PubMed; (b) F. Xuan, X. T. Luo and I. M. Hsing, Anal. Chem., 2013, 85, 4586 CrossRef CAS PubMed; (c) L. Shi, Z. Y. Chu, Y. Liu, W. Q. Jin and X. J. Chen, Biosens. Bioelectron., 2014, 54, 165 CrossRef CAS PubMed.
- (a) M. Zhang, L. Ge, S. Ge, M. Yan, J. Yu, J. Huang and S. Liu, Biosens. Bioelectron., 2013, 41, 544 CrossRef CAS PubMed; (b) W. H. Gao, A. Zhang, Y. S. Chen, Z. X. Chen, Y. W. Chen, F. S. Lu and Z. G. Chen, Biosens. Bioelectron., 2013, 49, 139 CrossRef CAS PubMed.
- (a) G. F. Wang, G. Xu, Y. H. Zhu and X. J. Zhang, Chem. Commun., 2014, 50, 747 RSC; (b) D. Li, A. Wieckowska and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 3927 CrossRef CAS PubMed; (c) Y. Hao, Q. Guo, H. Wu, L. Guo, L. Zhong, J. Wang, T. Lin, F. Fu and G. Chen, Biosens. Bioelectron., 2014, 52, 261 CrossRef CAS PubMed.
- J. Xu, J. Wu, C. Zong, H. X. Ju and F. Yan, Anal. Chem., 2013, 85, 3374 CrossRef CAS PubMed.
- (a) J. T. Groves, J. Porphyrins Phthalocyanines, 2000, 4, 350 CrossRef CAS; (b) R. Kuroda and H. Tanaka, J. Chem. Soc., Chem. Commun., 1994, 1575 RSC; (c) Y. Nitta and R. Kuroda, Biopolymers, 2006, 81, 376 CrossRef CAS PubMed.
- Y. Zhang, G. Mc. Zeng, L. Tang, Y. P. Li, Z. M. Chen and G. H. Huang, RSC Adv., 2014, 4, 18458 Search PubMed.
- X. H. Lou, T. Zhao, R. Liu, J. Ma and Y. Xiao, Anal. Chem., 2013, 85, 7574 CrossRef CAS PubMed.
- J. M. Burke, J. R. Kincaid and T. G. Spiro, J. Am. Chem. Soc., 1979, 6077 Search PubMed.
- J. Wan, G. Yin, X. J. Ma, L. Xing and X. L. Luo, Electroanalysis, 2014, 26, 823 CrossRef CAS.
- E. Sharon, X. Q. Liu, R. Freeman, O. Yehezkeli and I. Willner, Electroanalysis, 2013, 25, 851 CrossRef CAS.
- Y. Zhang, G. M. Zeng, L. Tang, Y. P. Li, Z. M. Chen and G. H. Huang, RSC Adv., 2014, 4, 18485 RSC.
- J. Y. Zhuang, L. B. Fu, D. P. Tang, M. D. Xu, G. N. Chen and H. H. Yang, Biosens. Bioelectron., 2013, 39, 315 CrossRef CAS PubMed.
- X. J. Yu, X. N. Liu, C. Q. Mou and Z. G. Wang, Luminescence, 2013, 28, 847 CrossRef CAS PubMed.
- L. X. Chen, N. Qi, X. K. Wang, L. Chen, H. Y. You and J. H. Li, RSC Adv., 2014, 4, 15055 RSC.
- H. Huang, J. J. Lv, D. L. Zhou, N. Bao, Y. Xu, A. J. Wang and J. J. Feng, RSC Adv., 2013, 3, 21691 RSC.
| This journal is © The Royal Society of Chemistry 2014 |


