Transient nature of graphene quantum dot formation via a hydrothermal reaction†
Abstract
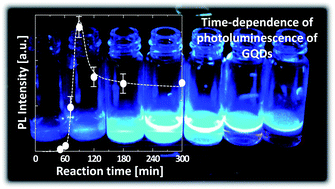
A facile, economic and environmentally friendly one-step approach for the preparation of highly luminescent graphene quantum dots (GQDs) was developed using a hydrothermal reaction between citric acid and urea. Unlike previous reports, we focused on the effect of the transient nature of GQD formation on the photoluminescence (PL) properties and molecular structure changes of the products. We found that the GQDs have an optimum reaction time and require an effective precursor to achieve excellent luminescent properties. The PL, ultraviolet-visible (UV-vis) absorption, zeta potential, and nuclear magnetic resonance (NMR) analyses of the GQDs prepared at various reaction times revealed that the molecular structures responsible for the luminescence of the GQDs are aggregates or condensation products of citric acid amides. We found that urea addition to the precursor drastically enhances the PL intensity of the GQDs, and it is 40 times higher than those prepared using the pure citric acid precursor. Additionally, a GQDs–polyvinyl alcohol composite achieved an excellent quantum yield (QY) of 43.6%.

 Please wait while we load your content...
Please wait while we load your content...