Study of captopril pharmacokinetics in rabbit blood with microdialysis based on online generated Au nanoclusters and pepsin–captopril interaction in luminol chemiluminescence
Kai Luoa,
Fei Niea,
Yumei Yanb,
Shixiang Wangb,
Xiaohui Zhengb and
Zhenghua Song*a
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry & Material Science, Northwest University, Xi’an, 710069, China. E-mail: songzhenghua@hotmail.com; zhsong123@nwu.edu.cn; Fax: +86-029-88302604; Tel: +86-029-88303798
bKey Laboratory of Resource Biology and Biotechnology in Western China, College of Life Sciences, Northwest University, Xi’an, 710069, China
First published on 6th November 2014
Abstract
A luminol–HAuCl4–pepsin (Pep) flow injection-chemiluminescence (FI-CL) system was explored to determine captopril (CAP) based on the CL intensity inhibition effect and applied to study CAP pharmacokinetics in rabbits with microdialysis. HAuCl4 and pepsin (Pep) could significantly enhance the luminol chemiluminescence (CL) intensity. It was found that sub-nanometre Au nanoclusters (AuNCs) were generated in the luminol–HAuCl4–Pep reaction solution. A possible mechanism for AuNCs generation is given. By means of the FI-CL and molecular docking (MD) methods, the Pep–CAP interaction was systematically studied. The results showed that CAP might enter into Pep active site Asp32 with the binding constant (K) 1.7 × 106 L mol−1, which could effectively inhibit the CL intensity. The CL intensity could be remarkably inhibited by CAP and the decrement of CL intensity was linearly correlated to the logarithm of CAP concentration in the range of 3.0 pmol L−1 to 0.1 μmol L−1, with a detection limit of 1.0 pmol L−1 (3σ). This proposed approach was successfully applied to determine CAP in rabbit’s blood during the 16 h after intragastric administration with an elimination ratio of 45.9% and recovery ratios from 89.0% to 112.0%. The pharmacokinetic results showed that CAP could be rapidly absorbed into blood with a peak concentration (Cmax) of 9.63 ± 1.45 μg mL−1 at a maximum peak time (Tmax) of 0.75 ± 0.08 h; the elimination half-life of 3.19 ± 0.13 h and the elimination rate constant of 7.27 ± 0.41 L g−1 h−1 in rabbits were derived, respectively.
Introduction
Protein–drug interactions have become a hot topic in the fields of medicine, chemistry and biology for drug discovery, screening, design and development.1–3 Recently, numerous works have been performed on predicting the binding sites of drugs to proteins and analyzing the interaction patterns between them.4,5 Pepsin (Pep) (MW: 34.5 kD) is a monomeric, two domain, mainly l-protein, with a high percentage of acidic residues (43 out of 327).6 The catalytic sites are Asp32 and Asp215 for the Pep to be active.7 Pep, as a digestive protease, is most efficient at cleaving peptide bonds between hydrophobic and aromatic amino acids such as phenylalanine (Phe), tryptophan (Trp) and tyrosine (Tyr). The interaction behavior of Pep with bisphenol A,8 nobiletin9 and fleroxacin10 was systemically investigated using fluorescence spectroscopy, UV-visible absorption, resonance light scattering, synchronous fluorescence spectroscopy, 3D spectroscopy and molecular docking (MD), while the relative interaction parameters, such as binding constants and thermodynamic parameters were given.Gold nanoclusters (AuNCs) have attracted substantial research interest in the fields of chemistry,11,12 materials,13,14 biology,15–17 and medicine.18 Considerable efforts have been devoted to exploring synthesis methods for stability, functionality and solubility of AuNCs.19–22 Synthesis methods for AuNCs with biological macromolecule-mediators such as DNA,23 peptides,24 bovine serum albumin (BSA)25 and Pep26 in alkaline solution have been reported. In order to overcome the time-consuming nature of AuNC biosynthesis, technologies such as microwaves27 and photolithography28 have gradually been applied in the biosynthesis of AuNCs. The photochemical induced effect (PCIE), as a photo-induced effect,29,30 could not only quickly induce AuNC generation in solution under biological macromolecule-mediation, but also endow AuNCs with some special photoelectric properties. In view of its special nature, PCIE will open a new way for the biosynthesis of AuNCs in solution. There are no reports of online generated AuNCs in a flow inject-chemiluminescence (FI-CL) system and the application on protein–drug interaction.
Captopril (CAP, Fig. 1) has a significant antihypertensive effect as an angiotensin converting enzyme inhibitor (ACEI), which could improve cardiac function in patients.31 The methods commonly used for determining CAP in vivo are liquid chromatography-mass spectrometry (LC-MS) or liquid chromatography-column derivatization-UV detection (LC-CD-VWD).32,33 But their time-consuming nature, expensive instrumentation and low sensitivities are bottlenecks of the above methods for CAP determination in vivo. CL methods have gradually become the general and practical methods for determining CAP with high sensitivity and wide dynamic range.34 Recently, Tzanavaras has reviewed a variety of flow related methods for CAP determination in both pharmaceutical and biological samples.35
It has been reported that HAuCl4 as a co-reactant can remarkably increase luminol CL intensity.36,37 To date, no flow inject-chemiluminescence (FI-CL) approach combined with HAuCl4 and Pep has been designed and developed for drug analysis in vivo and Pep–drug interaction. In this work, we developed a luminol–HAuCl4–Pep FI-CL approach for CAP determination, and applied the proposed approach to study CAP pharmacokinetics in rabbits with microdialysis. The aims of the present study were to: (1) investigate the mechanism of the complex enhancement effect of CL and the complex quench effect of CL in the luminol–HAuCl4–Pep/CAP CL system; (2) develop a luminol–HAuCl4–Pep FI-CL approach to study CAP pharmacokinetics in rabbits with microdialysis.
Experimental section
Chemicals and reagents
All the reagents used were analytical grade. Water was purified using a Milli-Q system (Millipore, Bedford, MA, USA) with a resistivity of 18.2 MΩ cm−1 and used throughout the whole experiment. Luminol (Fluka, Biochemika, Switzerland) and Pep (porcine gastric mucosa, 010M7006V, Sigma-Aldrich, St. Louis, MO, USA) were used without further purification. CAP was purchased from the National Institute of Control of Pharmaceutical and Biological Products, China. Chloroauric acid (HAuCl4, analytical grade) was purchased from Shanghai Reagent Factory, China; Capoten tablets (Sino-American Shanghai Squib Co., Ltd, China, H20010430) were purchased from a local dispensary.Stock solutions of CAP (1.0 mmol L−1) and Pep (100.0 μmol L−1) were prepared in purified water and stored at 4 °C. Working standard solutions of CAP and Pep were prepared daily by diluting the stock solution appropriately with purified water. A stock solution of luminol (2.5 × 10−2 mol L−1) was prepared by dissolving 0.44 g luminol in 100 mL NaOH (1.0 × 10−1 mol L−1) solution in a brown calibrated flask. A stock solution of HAuCl4 (2.5 × 10−2 mol L−1) was prepared by dissolving 1.0 g HAuCl4 in 100 mL purified water and stored at 4 °C.
Apparatus
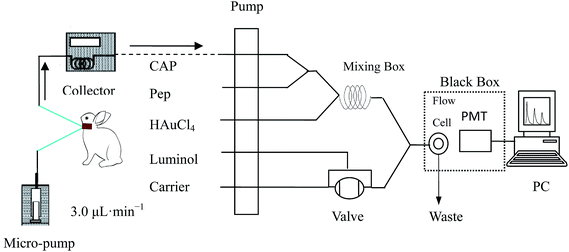
The apparatus (Model IFFM-E, Xi’an Remax Electronic Science-Tech. Co. Ltd) of the FI-CL system consisted of a sampling system, photomultiplier tube (PMT), and a PC with an IFFM-E client system (Remax, Xi’an, China). A polytetrafluoroethylene (PTFE) tube (1.0 mm i.d.) was used to carry the solutions. The microdialysis system was composed of a CMA/100 microinjection pump, a CMA/140 micro-fraction injector (CMA, Stockholm, Sweden) and microdialysis probes (CMA/20, Beijing Ying Bo Li Da Technology Development Co., Ltd, China). The UV-Vis absorption spectra (225–800 nm) were collected using a U-3010 spectrophotometer system (Hitachi, Japan). The TEM images were obtained using a Tecnai G2 F20 S-TWIJEM-2010 transmission electron microscope (FEI, USA) operated at 200 kV.The profile for different systems with static injection CL
The static injection CL method was used to evaluate the CL kinetics progress for different CL systems. Using the permutations method, four different FI-CL systems were designed to study the different CL mechanisms. For the luminol–dissolved oxygen/CAP CL system, 100 μL luminol solution was directly injected into the dissolved oxygen solution in the absence or presence of CAP. For the luminol–HAuCl4/CAP and luminol–Pep/CAP CL systems, 100 μL luminol solution was injected into the HAuCl4 solution and the Pep solution in the absence or presence of CAP, respectively. For the luminol–HAuCl4–Pep/CAP CL system, the Pep was first mixed with CAP, then with HAuCl4 to form the HAuCl4–Pep/CAP solution in the presence of CAP; finally 100 μL luminol solution was injected into the above HAuCl4–Pep/CAP solution. The CL intensity was measured by the PMT (negative voltage was set as 400 V) for 40 s.The procedure of luminol–HAuCl4–Pep CL combined with microdialysis
In the luminol–HAuCl4–Pep CL system, five flow lines were inserted into the solutions of luminol, carrier (purified water), HAuCl4, Pep and CAP, respectively, and the solutions were propelled by peristaltic pumps. Luminol (100 μL) was quantitatively injected into the mixed solution of HAuCl4, Pep and samples by six-way valve, then the mixture was delivered into the flow cell producing CL emission which was detected by PMT (negative voltage was set as 700 V). The concentration of CAP was quantified by the decrease of CL intensity (ΔI = I0 − Is), where Is and I0 are CL signals in the presence and in the absence of CAP samples, respectively.A retrograde calibration technique was used for the assessment of the in vivo recovery rate of the luminol–HAuCl4–Pep CL system (Fig. 2). Two hours post probe implantation, which served as a stabilization period, the perfuse (Cperf) and dialysate (Cdial) concentrations of CAP were determined using the luminol–HAuCl4–Pep FI-CL system. The relative loss of CAP during retro-dialysis (Lretro) or relative recovery (Rdial) by dialysis was then calculated as follows: Lretro = Rdial = (Cperf − Cdial)/Cperf.
 | ||
| Fig. 2 Scheme of CAP determination in rabbit blood of the luminol–HAuCl4–Pep FI-CL system with microdialysis. | ||
Molecular docking
The MD of Pep–CAP was performed with the open-free soft of Autodock 4.2 using a semi-flexible docking mode. The crystal structure of Pep (PDB entry 1YX9) was obtained from the Protein Data Bank. The 3D structure of CAP was generated using ChemDraw 10.0 and Chem3D 10.0 soft (Cambridge Soft, USA); and the energy-minimized conformation was obtained by the Gasteiger–Huckel charges with a gradient of 0.005 kcal mol−1.38 With the aid of AutoDock tools, the ligand root of CAP was detected and rotatable bonds were free-defined. The grid box with 60 Å × 60 Å × 60 Å along x, y, z axes of 0.375 Å spacing was set in the whole process of MD. The population size and the maximum number of energy evaluation were set as 1.5 × 102 and 2.5 × 106, respectively. The Lamarckian genetic algorithm was applied for docking simulations. The conformation with the lowest binding energy was analyzed using PyMOL 1.6.0.0.Method validation for CAP determination
The proposed method was validated regarding its selectivity, linearity, the limit of detection (LOD), accuracy, precision, recovery and stability. The linearity of methods were constructed between the relative CL intensity and the different concentrations of CAP. The LOD was considered as the final concentration that produced a signal-to-noise (S/N) ratio of 3. The precision and accuracy of the method were assessed by performing replicate analyses of CAP with anti-coagulant citrate dextrose (ACD) solution consisting of citric acid 3.5 × 10−3 mol L−1, sodium citrate 7.5 × 10−3 mol L−1, and dextrose 13.6 × 10−3 mol L−1. The precision was determined from inter-day and intra-day using six determinations of low, medium and high concentrations and expressed as relative standard deviation (RSD%). The extraction recovery rate was determined by calculating the ratio between the amounts of the drug-free samples and those spiked with known amounts of CAP into drug-free samples. The stability of the sample was assessed by measuring the analysis data of CAP standard samples with high, medium and low concentrations under ambient conditions. To evaluate its selectivity, different foreign species were added into a standard solution of CAP, and the impact of foreign substances on the standard CAP solution assessed.Pharmacokinetic study of CAP in rabbits
A Capoten tablet was stripped of the outer sugar coating and ground to a powder. The Capoten tablet powder (1.00 g) was accurately weighed and placed in a beaker, 50 mL deionized water was added with ultrasound for 30 min, and a constant volume was kept with purified water added to a 100 mL brown volumetric flask, chilled in the dark.Male rabbits (1.8–2.2 kg, n = 5) were purchased from the Laboratory Animal Center of Xi’an Jiaotong University (Xi’an, P.R. China) and housed in a cage with free access to food and water available ad libitum. The animals were acclimatized for at least one week with a 12 h light/dark cycle. All experimental rabbit surgery procedures were approved by the institutional animal experimentation committee of Xi’an Jiaotong University.
On the day of experiment, each rabbit was initially anesthetized with chloral hydrate solution (1.0 mg kg−1, subcutaneous) and catheters were positioned within the jugular vein toward the right atrium and then perfused with ACD solution. The flow rate of ACD was set at 3.0 μL min−1 by a microinjection pump for blood microdialysis. The rabbit’s body temperature was maintained at 37 °C with a heating blanket. Following a 2 h stabilization period after surgery, 1.16 mg kg−1 CAP was administered via intragastric (i.g.) administration. The dialysates were collected every 25 min for 16 h and preserved at −4 °C in a refrigerator. The concentration of CAP in the dialysate was determined by the luminol–HAuCl4–Pep FI-CL system. CAP microdialysis concentration (Cm) was converted to unbound concentration (Cu) as follows: Cu = Cm/Lretro.
Results and discussion
Relative CL intensity–time profile
The relative CL intensity–time profiles of different photochemical reaction systems are shown in Fig. 3. It can be seen from the CL intensity–time profiles that the maximum time (Tmax) for reaching maximum CL intensity (Imax) of the luminol–dissolved oxygen and luminol–HAuCl4 CL systems (curves 2 and 6) was 3.0 s with the Imax values of 45 and 550, respectively; compared with the luminol–dissolved oxygen and luminol–HAuCl4 CL system, the Tmax of the luminol–Pep and luminol–HAuCl4–Pep CL systems (curves 4 and 8) were shortened from 3.0 s to 2.8 s, and the corresponding CL intensities for curves 4 and 8 were 104 and 1143, respectively. From these results, we could speculate that Pep could accelerate the electron transfer rate due to the proton process of luminol or luminol–HAuCl4 and Pep in alkaline solution, which could lead to the shortage of Tmax for curves 4 and 8. Compared with the CL system of curves 2 and 4, HAuCl4 as the co-reactant could remarkably increase the luminol CL intensity. The reason might be attributed to the Au nuclei generated in the alkaline solution, which could cause the quantum confinement effect mediated by the PCIE of luminol. In the presence of CAP (CCAP = 10.0 pmol L−1), it could sharply quench from 1143 to 1017 for the luminol–HAuCl4–Pep CL system with a quenching ratio of 11.0%. The CL systems of luminol–dissolved oxygen, luminol–HAuCl4 and luminol–Pep had almost no or slightly inhibitory effect. This result could explain why the luminol–HAuCl4–Pep CL system had a higher sensitivity for minor changes in the confirmation of Pep, which was mediated by the interaction between Pep and CAP. Interesting, the CL intensity for curves 1–6 extinguished during 40 s, while curves 7 and 8 had more stable CL intensities than other CL intensities and lasted for 80 s before extinguishing.The CL intensity could be enhanced and inhibited when alkaline luminol was mixed with different HAuCl4–Pep solution and HAuCl4–Pep/CAP solution. Different CL response intensities could be obtained (Fig. 3). But of all the mentioned CL systems, the luminol–HAuCl4–Pep and luminol–HAuCl4–Pep/CAP CL intensities could be significantly enhanced and inhibited compared with other CL systems. The mechanisms of luminol–dissolved oxygen, luminol–Pep and luminol–HAuCl4 have been explained in previous reports.39,40 Here we mainly focused on the luminol–HAuCl4–Pep and luminol–HAuCl4–Pep/CAP CL systems to explain the possible mechanism of AuNCs generated in alkaline solution and the interaction of Pep/CAP in the luminol–HAuCl4–Pep CL system.
CL mechanism for luminol–HAuCl4–Pep/CAP system
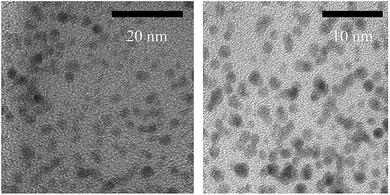 | ||
| Fig. 4 HR-TEM images of sub-nanometre AuNCs generated in the luminol–HAuCl4–pepsin CL reaction solution. | ||
 | ||
| Fig. 5 UV-Vis graphic of the luminol–HAuCl4–Pep system with a pH of 10.5. The corresponding concentrations of luminol, HAuCl4 and Pep were 2.5 × 10−4, 2.5 × 10−5 and 1.0 × 10−6 mol L−1, respectively. | ||
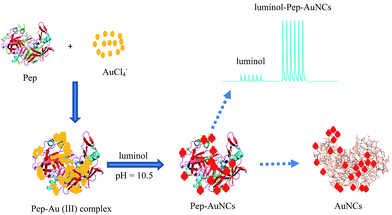
Combined with the results of TEM and UV-Vis, in the profile of the luminol–HAuCl4–Pep CL system, it was found that Pep–Au(III) complex was firstly formed on mixing Pep with HAuCl4; then this was reduced on addition into alkaline luminol solution (Fig. 6). The detailed mechanism could refer to our previous report about photochemically induced formation of Au nanomaterial with size and shape controlled by the luminol–Pep CL reaction.44 The AuNCs generated in the luminol–HAuCl4–Pep CL system were conducted in the fixed alkaline luminol solution with a pH value of 10.5. From the perspective of the experimental performance, the alkaline luminol had the optimal luminous efficiency at a pH of 10.5, which could improve the detection sensitivity. Due to their properties of sub-nanometre size and good hydrophilicity, AuNCs could effectively prevent pipeline blockage, which is caused by the effect of deposition and aggregation of the AuNCs, to disturb the sensitivity and reproducibility for CAP determination.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Pep–CAP complex was formed. The thermodynamic parameters of CAP to Pep were calculated using the van’t Hoff equation.46 The results indicated that ΔHo > 0, ΔSo > 0 and ΔGo < 0 at different temperatures (Table 1). It could be deduced that the binding force was mainly on the hydrophobic interaction.47 The MD studies could give some insight into the protein–drug interactions.48,49 In the presence of CAP, the docked conformation of Pep/CAP is shown in Fig. 7. The docked pose shows that CAP might enter into the active site cavity of Pep and form a hydrogen bond to Asp32 with the bond distance of 3.2 Å. For the Pep/CAP complex, the inhibition constants, free energy of binding and accessible surface area (ASA) were 8.7 × 105 L mol−1, −30.44 kJ mol−1 and 119.38 Å2, respectively (Table 2).
1 Pep–CAP complex was formed. The thermodynamic parameters of CAP to Pep were calculated using the van’t Hoff equation.46 The results indicated that ΔHo > 0, ΔSo > 0 and ΔGo < 0 at different temperatures (Table 1). It could be deduced that the binding force was mainly on the hydrophobic interaction.47 The MD studies could give some insight into the protein–drug interactions.48,49 In the presence of CAP, the docked conformation of Pep/CAP is shown in Fig. 7. The docked pose shows that CAP might enter into the active site cavity of Pep and form a hydrogen bond to Asp32 with the bond distance of 3.2 Å. For the Pep/CAP complex, the inhibition constants, free energy of binding and accessible surface area (ASA) were 8.7 × 105 L mol−1, −30.44 kJ mol−1 and 119.38 Å2, respectively (Table 2).
| Temperature/K | K/L mol−1 | n | ΔH/kJ mol−1 | ΔS/J mol−1 K−1 | ΔG/kJ mol−1 |
|---|---|---|---|---|---|
| 283 | 8.1 × 105 | 0.67 | −32.03 | ||
| 288 | 9.8 × 105 | 0.75 | −33.02 | ||
| 293 | 1.3 × 106 | 0.84 | 24.48 | 199.68 | −34.03 |
| 298 | 1.7 × 106 | 0.87 | −35.02 | ||
| 313 | 2.2 × 106 | 0.89 | −38.02 |
| FI-CL | Molecular docking | ||
|---|---|---|---|
| n | 0.87 | Binding site (n) | Asp32 |
| KE (L mol−1) | 1.7 × 106 | Bind distance (Å) | 3.2 |
| ΔHo (kJ mol−1) | 24.48 | KM (L mol−1) | 8.7 × 105 |
| ΔSo (kJ mol−1 K−1) | 199.68 | ASA (Å2) | 119.38 |
| ΔGo (298.13 K) (kJ mol−1) | −35.03 | ΔGo (298.13 K) (kJ mol−1) | −30.44 |
In the luminol–HAuCl4–Pep/CAP CL system, CAP solution was first mixed with Pep solution under neutral conditions, and then mixed successively with HAuCl4 solution, and alkaline luminol solution (pH = 10.5). In the profile of the luminol–HAuCl4–Pep/CAP CL system, it was found that the CL intensity of luminol–HAuCl4–Pep/CAP could effectively be inhibited compared with the CL intensity of luminol–HAuCl4–Pep. The reason might be attributed to the Pep–CAP interaction. In the Pep conformation, the Asp32 is a negatively charged polar residue located on the interface cleft of Pep.50 For the structure of CAP, the thiol group (–SH) and carboxyl group (–COOH) make it easy to form a hydrophilic microenvironment due to the strong electron-withdrawing effect. In the Pep/CAP complex (Fig. 7), the O atom of the carbonyl group in CAP could bind to the Asp32 of Pep with a hydrogen bond and enter into the hydrophilic center of Pep, which is formed by the two activity sites, Asp32 and Asp215 on each cleft of Pep. The interaction of Asp32 and the carbonyl group was accessible to protonation between Pep and CAP. The thiol group of CAP as the special group plays a key function for drug-efficacy in vivo. The thiol group in the Pep/CAP complex had no binding with other groups of Pep. These results indicate that the bioactivity of CAP is not influenced by the activity center of Pep, when in the process of protein–drug interaction between Pep and CAP. The flexible loop formed with Asp32 and Asp215, which is commonly known as the “flap”, could induce the conformational change of Pep.51 On Pep–CAP interaction, the amino acid residues on the Pep surface such as Asp11, Asp159, Glu4, Glu13 and Asp118 would be embedded into the interior of Pep. The abnormally high pKa values of Asp11 and Asp159 gradually tended to normal value due to the protonation process between Pep and CAP. This change would directly promote reduction potential reduction, which could inhibit Au atom aggregation on the surface of Pep with other amino acid residues, produce the CL quenching effect.
In short, the possible mechanism for producing sub-nanometre AuNCs, enhancing the CL intensity and inhibiting the CL intensity on adding CAP might be as follows:
(1) Under alkaline conditions, Au3+ and negatively charged amino acid residues could form a Pep–Au3+ complex on the surface of Pep. Due to the unstable conformation of Pep in alkaline solution, the negatively charged polar residues with abnormally high pKa values could promote Au3+ flow into the vicinity of negatively charged amino acid residues on the principle of charge density matching and form sub-nanometre AuNCs.52,53 Meanwhile, microscopic changes of Pep confirmation could accelerate the electron transfer rate of excited 3-aminophthalate, giving the enhancement of the CL intensity of luminol and producing the complex enhancement effect of CL (CEC).
(2) CAP could bind to Asp32 with a hydrogen bond, and enter into the hydrophilic center of Pep, leading to Pep’s conformational change, reducing the reduction potential on Pep’s surface. Based on the cascading effect of Pep/CAP interaction, the electron transfer rate of excited 3-aminophthalate was inhibited, and produced the complex quench effect of CL (CQC).
| Optimum factors | Optimum range | Final setting |
|---|---|---|
| Luminol | 5.0 × 10−7 to 2.5 × 10−4 mol L−1 | 2.5 × 10−4 mol L−1 |
| NaOH | 5.0 × 10−3 to 2.0 × 10−1 mol L−1 | 2.5 × 10−2 mol L−1 |
| HAuCl4 | 1.0 × 10−6 to 1.5 × 10−4 mol L−1 | 2.5 × 10−5 mol L−1 |
| Pep | 1.0 × 10−10 to 1.0 × 10−6 mol L−1 | 1.0 × 10−6 mol L−1 |
| Flow rate | 1.0–5.0 mL min−1 | 2.0 mL min−1 |
| Mixing tube | 5–20 cm | 10 cm |
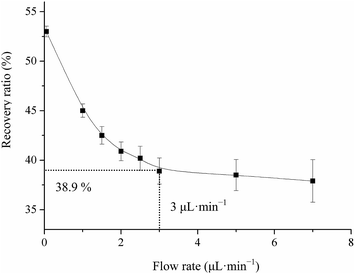 | ||
| Fig. 8 Relationship curve between flow rate and recovery ratio for a fixed concentration of CAP (210.0 pg mL−1) in the microdialysis system. | ||
It is well known that the efficiency of microdialysis is mainly controlled by some distinct factors, which include surface area of the dialysis membrane, molecule cut-off rate and CAP concentration. The length of the blood probe membrane was 2.5 cm and the molecular weight of CAP was 217.29, which was much less than the maximum cut-off limitation (3.5 kD). To evaluate the in vivo recovery, the microdialysis probe was placed in three unknown concentrations of CAP blood (CAP standard solution + blank serum) (2.1, 21.0, 210.0 and 2100.0 pg mL−1) with 3.0 μL min−1 of ACD solution for microdialysis to determine the factual CAP concentration according to the linear relationship of the luminol–HAuCl4–Pep CL system. The results of average recoveries of three different CAP concentrations were 36.8 ± 0.02, 38.9 ± 0.05, 41.2 ± 0.08 and 42.8 ± 0.13% in blood, respectively. The data indicated that the recovery for microdialysis probes in blood shared no significant differences among the concentration ranges of 2.1, 21.0, 210.0 and 2100.0 pg mL−1. These results suggest that the recovery for microdialysis probes is concentration independent, which could be applied to the study of CAP pharmacokinetics in rabbits.
Method validation
| FI-CL | Linear equation | Linear range | LOD | R2 |
|---|---|---|---|---|
| Dissolved oxygen | ΔI = 11.205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln C + 60.96 ln C + 60.96 |
10.0 nM to 30 μM | 3.0 nM | 0.998 |
| Pep | ΔI = 35.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 948.5 C + 948.5 |
7.0 pM to 3.0 nM | 2.3 pM | 0.983 |
| HAuCl4 | ΔI = 53.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 1445.2 C + 1445.2 |
30.0 pM to 100.0 nM | 10.0 pM | 0.988 |
| HAuCl4–Pep | ΔI = 125.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 3310.2 C + 3310.2 |
3.0 pM to 0.1 μM | 1.0 pM | 0.995 |
| Time day | Io | RSD% | Is (2.1)/pg mL−1 | RSD% | Is (21.0)/pg mL−1 | RSD% | Is (210.0)/pg mL−1 | RSD% |
|---|---|---|---|---|---|---|---|---|
| 1st | 1132 | 1.5 | 1004 | 1.6 | 715 | 2.6 | 426 | 3.8 |
| 2nd | 1125 | 1.8 | 997 | 2.3 | 708 | 3.1 | 419 | 3.5 |
| 3rd | 1122 | 1.9 | 994 | 2.6 | 705 | 3.2 | 416 | 4.1 |
| 4th | 1135 | 2.1 | 1007 | 2.3 | 718 | 2.3 | 429 | 4.3 |
| 5th | 1130 | 2.3 | 1002 | 2.0 | 713 | 2.5 | 424 | 4.6 |
| QC samples/pg mL−1 | Precision (n = 5) | ACD solution (n = 5) | Blank dialysis (n = 5) | |||
|---|---|---|---|---|---|---|
| Inter-day | Intra-day | Found | Recovery | Found | Recovery | |
| 2.10 | 2.08 ± 0.12 | 2.06 ± 0.22 | 2.08 ± 0.12 | 99.04% | 2.02 ± 0.19 | 96.19% |
| 21.0 | 20.32 ± 1.69 | 20.09 ± 2.89 | 20.32 ± 1.69 | 96.76% | 20.04 ± 1.95 | 95.42% |
| 210.0 | 202.87 ± 3.89 | 196.87 ± 4.78 | 202.87 ± 3.89 | 96.60% | 200.96 ± 4.61 | 95.69% |
In order to eliminate disturbance of the dialysate and improve the sensitivity of CAP determination in rabbit’s blood dialysate, the interference of blank blood dialysate was tested by diluting serial blank blood dialysate with purified water to gain similar intensity to that of the luminol–HAuCl4–Pep CL. In the presence of blank blood dialysate, the relative CL intensity in the luminol–HAuCl4–Pep system was approximately equal to the CL intensity in the absence of blank blood while diluted 5 × 103 times with purified water.
| Interval/h | Added/found pg mL−1 | RSD% | Recovery% | Content/ng mL−1 | Eliminate% |
|---|---|---|---|---|---|
| a 2.32 mg CAP was administered i.g. to rabbit (2.0 kg). | |||||
| 0.00 | 0/— | — | 99.3 | — | — |
| 2.10/2.02 | 0.5 | ||||
| 0.08 | 0/3.15 | 0.9 | 98.28 | 18.53 ± 0.83 | 0.01 |
| 21.00/24.09 | 0.6 | ||||
| 0.16 | 0/28.51 | 2.8 | 106.43 | 40.07 ± 0.84 | 0.19 |
| 21.0/51.34 | 1.2 | ||||
| 0.25 | 0/78.42 | 2.3 | 118.43 | 59.51 ± 0.79 | 0.38 |
| 210.0/302.87 | 1.2 | ||||
| 0.50 | 0/172.55 | 1.8 | 96.29 | 171.22 ± 0.72 | 0.89 |
| 210.0/376.15 | 1.8 | ||||
| 0.75 | 0/151.84 | 2.7 | 107.38 | 411.55 ± 0.53 | 1.87 |
| 210.0/373.05 | 1.9 | ||||
| 1.00 | 0/129.7 | 1.9 | 99.44 | 1003.59 ± 0.47 | 8.02 |
| 210.0/339.00 | 3.2 | ||||
| 1.50 | 0/103.12 | 1.6 | 105.17 | 1595.53 ± 0.09 | 12.93 |
| 210.0/318.45 | 2.5 | ||||
| 2.00 | 0/83.39 | 1.9 | 104.44 | 1277.57 ± 0.14 | 9.14 |
| 210.0/297.09 | 3.3 | ||||
| 3.00 | 0/57.83 | 1.6 | 100.10 | 942.46 ± 0.29 | 4.38 |
| 210.0/267.89 | 4.1 | ||||
| 4.00 | 0/36.26 | 2.8 | 109.09 | 661.09 ± 0.35 | 3.12 |
| 21.0/60.56 | 3.7 | ||||
| 5.00 | 0/23.58 | 2.6 | 109.57 | 460.35 ± 0.39 | 2.26 |
| 21.0/46.84 | 3.4 | ||||
| 6.00 | 0/7.20 | 2.9 | 115.82 | 320.30 ± 0.44 | 1.42 |
| 2.1/10.44 | 4.6 | ||||
| 8.00 | 0/3.97 | 4.1 | 107.17 | 155.87 ± 0.58 | 0.73 |
| 2.1/6.35 | 4.2 | ||||
| 10.00 | 0/1.16 | 3.5 | 113.97 | 75.18 ± 0.63 | 0.45 |
| 2.1/3.42 | 3.2 | ||||
| 12.00 | 0/0.63 | 2.8 | 95.14 | 36.45 ± 0.87 | 0.26 |
| 2.1/2.70 | 4.0 | ||||
| 14.00 | 0/0.44 | 3.5 | 98.53 | 18.54 ± 0.89 | 0.14 |
| 2.1/2.53 | 4.3 | ||||
| 16.00 | 0/0.31 | 3.6 | 100.38 | 8.15 ± 1.02 | 0.05 |
| 2.1/2.41 | 2.8 | ||||
| Total elimination rate | 45.9 | ||||
 | ||
| Fig. 9 CAP concentration–time fitting curves for 5 rabbits after intragastric (i.g.) administration. | ||
The pharmacokinetic parameters for CAP in dialysate were obtained with the aid of DAS soft. Various parameters such as area under curve (AUC), peak plasma concentration (Cmax), time to reach the peak (Tmax), and elimination rate constant (Kel), absorption and elimination half-life (T1/2), the total mean residence time (MRT) and absorption efficiency were calculated for each rabbit. AUC0–∞ was calculated as AUC0–t + Clast/Kel, where Clast is the last measurable concentration. The volume of distribution was obtained as dose/AUC0–∞. The mean pharmacokinetic parameters for 5 rabbits are listed in Table 8. The results showed that the maximum peak concentration of CAP was 9.63 ± 1.45 μg mL−1 at 0.75 ± 0.08 h and a detectable quantity (6.23 ± 0.4 pg mL−1) was found at 16 h after its i.g. administration, which was higher than the limit of quantity (LOQ) of the analytical approach. Meanwhile, the value of the higher AUC0–t (613.16 ± 5.09 mg L−1 h−1), V1/F (2.02 ± 0.17 L g−1) and lower MRT0–t time (12.47 ± 0.39 h) showed that CAP has a strong ability to penetrate biological membranes and is mainly distributed in the rich blood of organs and tissues in vivo, excreted with the prototype with CL/F (7.27 ± 0.41 L g−1 h−1). After a single i.g. of CAP, it could rapidly be absorbed from the gastro-intestinal tract with peak blood level of 0.8 μg mL−1 in about an hour period with the minimal absorption of 75%. Up to 50% of CAP was metabolized through the liver, oxidized to its respective disulfides, and the remaining was excreted in the urine.58,59 The results showed that CAP could be in line with the two-compartment open model in vivo.
| Parameters | Sample no. | |||||
|---|---|---|---|---|---|---|
| 1# | 2# | 3# | 4# | 5# | M ± SD | |
| a AUC0–t, under the curve up to the last time (t); Tmax, the time to reach peak concentration; Cmax, the maximum plasma concentration; V1/F, the apparent volume of distribution; K, the apparent rate constant; t1/2α and t1/2β, the apparent absorption and elimination half-life; MRT0–t, mean residence time. | ||||||
| t1/2α (h) | 0.187 | 0.203 | 0.219 | 0.196 | 0.256 | 0.212 ± 0.07 |
| t1/2β (h) | 3.56 | 3.17 | 3.24 | 2.88 | 3.11 | 3.192 ± 0.13 |
| V1/F (L g−1) | 3.24 | 2.84 | 3.02 | 3.15 | 2.87 | 2.02 ± 0.17 |
| CL/F (L g−1 h−1) | 8.12 | 7.01 | 6.35 | 7.24 | 7.63 | 7.27 ± 0.41 |
| AUC0–t (mg L−1 h−1) | 569.42 | 486.13 | 721.36 | 688.29 | 600.58 | 613.16 ± 5.09 |
| Tmax (h) | 0.79 | 0.72 | 0.76 | 0.75 | 0.71 | 0.75 ± 0.08 |
| Cmax (μg mL−1) | 7.34 | 8.18 | 9.24 | 11.36 | 11.02 | 9.63 ± 1.45 |
| MRT0–t (h) | 12.36 | 11.45 | 13.27 | 12.22 | 13.06 | 12.47 ± 0.39 |
Statistical analysis of the main pharmacokinetic parameters, such as AUC0–t, V1/F, MRT0–t, CL/F and Cmax was performed using one-way analysis of variance (ANOVA) followed by Spss19.0 soft. The statistical results showed that the pharmacokinetic parameters had no significant specific difference (P > 0.05).
Conclusions
Based on the luminol CL intensity enhanced by Pep and HAuCl4, a luminol–HAuCl4–Pep FI-CL system was constructed for the first time. In the proposed FI-CL system, sub-nanometre AuNCs were generated in the luminol–HAuCl4–Pep CL reaction solution, which could potentially enhance the CL intensity; a possible mechanism for enhancing the CL intensity with HAuCl4 and Pep was given. This proposed FI-CL approach was successfully applied to study the CAP pharmacokinetics in rabbits with microdialysis. The results showed that CAP has a Cmax (9.63 ± 1.45 μg mL−1) at Tmax (0.75 ± 0.08 h), corresponding to t1/2β (3.19 ± 0.13 h) and CL/F (7.27 ± 0.41 L g−1 h−1) in rabbits in vivo. The pharmacokinetic results showed that CAP fits a two-compartment open model in rabbits.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21275118) and the Open Fund from the Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education.References
- J. P. Renaud and M. A. Delsuc, Curr. Opin. Pharmacol., 2009, 9, 622–628 CrossRef CAS PubMed
.
- W. F. J. de Azevedo, R. A. Caceres, I. Pauli, L. F. Timmers, G. B. Barcellos, K. B. Rocha and M. B. Soares, Curr. Drug Targets, 2009, 10, 271–278 CrossRef
.
- S. M. Huang, H. Zhao, J. I. Lee, K. Reynolds, L. Zhang, R. Temple and L. J. Lesko, Clin. Pharmacol. Ther., 2010, 87, 497–503 CrossRef CAS PubMed
.
- R. Minai, Y. Matsuo, H. Onuki and H. Hirota, Proteins: Struct., Funct., Bioinf., 2008, 72, 367–381 CrossRef CAS PubMed
.
- J. Colinge, U. Rix, K. L. Bennett and G. Superti-Furga, Proteomics Clin. Appl., 2012, 6, 102–116 CrossRef CAS PubMed
.
- B. M. Dunn, Chem. Rev., 2002, 102, 4431–4458 CrossRef CAS PubMed
.
- D. R. Dee and R. Y. Yada, Biochemistry, 2010, 49, 365–371 CrossRef CAS PubMed
.
- H. M. Zhang, J. Cao, Z. H. Fei and Y. Q. Wang, J. Mol. Struct., 2012, 1021, 34–39 CrossRef CAS PubMed
.
- H. J. Zeng, T. T. Qi, R. Yang, J. You and L. B. Qu, J. Fluoresc., 2014, 24, 1031–1040 CrossRef CAS PubMed
.
- S. Q. Lian, G. R. Wang, L. P. Zhou and D. Z. Yang, Luminescence, 2013, 28, 967–972 CrossRef CAS PubMed
.
- H. J. Zhang, T. Watanabe, M. Okumura, M. Haruta and N. Toshima, Nat. Mater., 2011, 11, 49–52 CrossRef PubMed
.
- H. Häkkinen, S. Abbet, A. Sanchez, U. Heiz and U. Landman, Angew. Chem., Int. Ed., 2003, 42, 1297–1300 CrossRef PubMed
.
- W. Chen and S. W. Chen, Angew. Chem., Int. Ed., 2009, 48, 4386–4389 CrossRef CAS PubMed
.
- Z. W. Wang and R. E. Palmer, Nano Lett., 2012, 12, 91–95 CrossRef CAS PubMed
.
- C. A. J. Lin, T. Y. Yang, C. H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J. L. Shen, H. H. Wang and H. I. Yeh, ACS Nano, 2009, 3, 395–401 CrossRef CAS PubMed
.
- S. Palmal and N. R. Jana, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2014, 6, 102–110 CrossRef CAS PubMed
.
- H. H. Wang, C. A. J. Lin, C. H. Lee, Y. C. Lin, Y. M. Tseng, C. L. Hsieh, C. H. Chen, C. H. Tsai, C. T. Hsieh and J. L. Shen, ACS Nano, 2011, 5, 4337–4344 CrossRef CAS PubMed
.
- W. Y. Chen, J. Y. Lin, W. J. Chen, L. Y. Luo, D. E. Wei-Guang and Y. C. Chen, Nanomedicine, 2010, 5, 755–764 CrossRef CAS PubMed
.
- Z. K. Wu, M. A. MacDonald, J. Chen, P. Zhang and R. C. Jin, J. Am. Chem. Soc., 2011, 133, 9670–9673 CrossRef CAS PubMed
.
- C. L. Liu, H. T. Wu, Y. H. Hsiao, C. W. Lai, C. W. Shih, Y. K. Peng, K. C. Tang, H. W. Chang, Y. C. Chien and J. K. Hsiao, Angew. Chem., Int. Ed., 2011, 50, 7056–7060 CrossRef CAS PubMed
.
- R. C. Jin, H. F. Qian, Z. K. Wu, Y. Zhu, M. Z. Zhu, A. Mohanty and N. Garg, J. Phys. Chem. Lett., 2010, 1, 2903–2910 CrossRef CAS
.
- Z. K. Wu and R. C. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed
.
- G. Y. Liu, Y. Shao, K. Ma, Q. H. Cui, F. Wu and S. J. Xu, Gold Bull., 2012, 45, 69–74 CrossRef CAS PubMed
.
- J. George and K. G. Thomas, J. Am. Chem. Soc., 2010, 132, 2502–2503 CrossRef CAS PubMed
.
- J. P. Xie, Y. G. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed
.
- H. Kawasaki, K. Hamaguchi, I. Osaka and R. Arakawa, Adv. Funct. Mater., 2011, 21, 3508–3515 CrossRef CAS
.
- L. Yan, Y. Q. Cai, B. Z. Zheng, H. Y. Yuan, Y. Guo, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 1000–1005 RSC
.
- H. Yabu, Chem. Commun., 2011, 47, 1196–1197 RSC
.
- S. H. Lee, A. G. Larsen, K. Ohkubo, Z. L. Cai, J. R. Reimers, S. Fukuzumi and M. J. Crossley, Chem. Sci., 2012, 3, 257–269 RSC
.
- H. Zeng, X. W. Du, S. C. Singh, S. A. Kulinich, S. Yang, J. He and W. Cai, Adv. Funct. Mater., 2012, 22, 1333–1353 CrossRef CAS
.
- R. F. Popovici, E. M. Seftel, G. D. Mihai, E. Popovici and V. A. Voicu, J. Pharm. Sci., 2011, 100, 704–714 CrossRef CAS PubMed
.
- W. M. M. Mahmoud and K. Kümmerer, Chemosphere, 2012, 88, 1170–1177 CrossRef CAS PubMed
.
- N. Rastkari, M. Khoobi, A. Shafiee, M. R. Khoshay and R. Ahmadkhaniha, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 932, 144–151 CrossRef CAS PubMed
.
- P. Khan, D. Idress, M. A. Moxley, J. A. Corbett, F. Ahmad, G. V. Figura, W. S. Sly, A. Waheed and M. I. Hassan, Appl. Biochem. Biotechnol., 2014, 173, 333–355 CrossRef CAS PubMed
.
- P. D. Tzanavaras, Anal. Lett., 2011, 44, 560–576 CrossRef CAS
.
- M. Iranifam, TrAC, Trends Anal. Chem., 2013, 51, 51–70 CrossRef CAS PubMed
.
- Q. Q. Li, L. J. Zhang, J. G. Li and C. Lu, TrAC, Trends Anal. Chem., 2011, 30, 401–413 CrossRef CAS PubMed
.
- J. Wang, S. H. Zhang, J. Zhang, P. Ren, Y. C. Chen, J. H. Li, W. Wang, Y. Ma, R. F. Shi and C. H. Wang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1605–1609 CrossRef CAS PubMed
.
- X. J. Tan, Z. M. Wang, D. H. Chen, K. Luo, X. Y. Xiong and Z. H. Song, Chemosphere, 2014, 108, 26–32 CrossRef CAS PubMed
.
- Z. F. Zhang, H. Cui, C. Z. Lai and L. J. Liu, Anal. Chem., 2005, 77, 3324–3329 CrossRef CAS PubMed
.
- Z. Wu, M. A. MacDonald, J. Chen, P. Zhang and R. Jin, J. Am. Chem. Soc., 2011, 133, 9670–9673 CrossRef CAS PubMed
.
- Y. S. Chen and P. V. Kamat, J. Am. Chem. Soc., 2014, 136, 6075–6082 CrossRef CAS PubMed
.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed
.
- K. Luo, X. H. Zheng and Z. H. Song, RSC Adv., 2014, 4, 45230–45233 RSC
.
- Z. M. Wang and Z. H. Song, Analyst, 2010, 135, 2546–2553 RSC
.
- O. Cañadas and C. Casals, Methods Mol. Biol., 2013, 974, 55–71 Search PubMed
.
- X. Y. Gao, Y. C. Tang, W. Q. Rong, X. P. Zhang, W. J. Zhao and Y. Q. Zi, Am. J. Anal. Chem., 2011, 2, 250–257 CrossRef CAS
.
- Y. Chen and B. K. Shoichet, Nat. Chem. Biol., 2009, 5, 358–364 CrossRef CAS PubMed
.
- A. S. El-Azab, M. A. Al-Omar, A. A. M. Abdel-Aziz, N. I. Abdel-Aziz, M. A. A. El-Sayed, A. M. Aleisa, M. M. Sayed-Ahmed and S. G. Abdel-Hamide, Eur. J. Med. Chem., 2010, 45, 4188–4198 CrossRef CAS PubMed
.
- D. Dee, J. Pencer, M. P. Nieh, S. Krueger, J. Katsaras and R. Y. Yada, Biochemistry, 2006, 45, 13982–13992 CrossRef CAS PubMed
.
- J. R. Engen, Anal. Chem., 2009, 81, 7870–7875 CrossRef CAS PubMed
.
- J. W. Liu, TrAC, Trends Anal. Chem., 2014, 58, 99–111 CrossRef CAS PubMed
.
- J. M. Slocik, A. O. Govorov and R. R. Naik, Nano Lett., 2011, 11, 701–705 CrossRef CAS PubMed
.
- H. Albrecht, Z. Phys. Chem., 1928, 136, 321–330 Search PubMed
.
- P. Nandi and S. M. Lunte, Anal. Chim. Acta, 2009, 651, 1–14 CrossRef CAS PubMed
.
- H. Ronald Zielke, C. L. Zielke and P. J. Baab, J. Neurochem., 2009, 109, 24–29 CrossRef PubMed
.
- D. Feuerstein, A. Manning, P. Hashemi, R. Bhatia, M. Fabricius, C. Tolias, C. Pahl, M. Ervine, A. J. Strong and M. G. Boutelle, J. Cereb. Blood Flow Metab., 2010, 30, 1343–1355 CrossRef CAS PubMed
.
- A. Luczak, R. Zakrzewski and M. Dobrogowski, Bioanalysis, 2012, 4, 1481–1489 CrossRef CAS PubMed
.
- G. Donáth-Nagy, S. Vancea and S. Imre, Croat. Chem. Acta, 2011, 84, 423–427 CrossRef
.
| This journal is © The Royal Society of Chemistry 2014 |