Molecular mechanism of action of K(D)PT as an IL-1RI antagonist for the treatment of rhinitis
Chanjuan Lia,
Hu Gea,
Lujia Cuib,
Yali Lia,
Bao Chenga,
Guodong Zhanga,
Ziying Zhanga,
Hao Qia,
Yan Ruanb,
Qiong Gu*a and
Jun Xu*a
aSchool of Pharmaceutical Sciences & Institute of Human Virology, Sun Yat-Sen University, 132 East Circle Road at University City, Guangzhou, 510006, China. E-mail: junxu@biochemomes.com
bNo. 1 Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, 510006, China
First published on 24th September 2014
Abstract
Background: Interleukin-1 receptor type I (IL-1RI) is critical for both innate immunity and inflammation. IL-1RI stimulates thymocyte proliferation and the release of several interleukin cytokines. These properties have increased interest in targeting IL-1RI for the treatment of inflammatory diseases. Here, an IL-1RI antagonist, K(D)PT (Lys-D-Pro-Thr), was tested in an allergic rhinitis model. The mechanism of action was then investigated. Methods: HEK293/IL-1RI cells and an allergic rhinitis animal model were treated with K(D)PT to evaluate its therapeutic effects. Fifty nanosecond (ns) molecular dynamic (MD) simulations were performed on the K(D)PT/IL-1RI complex, unliganded IL-1RI, and the IL-1β/IL-1RI complex to explore the mechanism of action of K(D)PT. Results: K(D)PT down-regulated the IL-1RI-mediated induction of IL-2 and IL-4 mRNA expression by IL-1β in HEK293/IL-1RI cells. In addition, nose itching was alleviated in mice treated with K(D)PT. Serum levels of IL-2 and IL-4 as well as eosinophil infiltration were also reduced. The data suggested that IL-1RI was highly expressed in the nasal mucosa of mice with allergic rhinitis. MD simulations revealed the following: (1) IL-1RI remains in the open conformation in the IL-1RI/IL-1β complex; (2) in unliganded IL-1RI, domains I and III randomly moved closer and apart without any significant energetic changes; (3) K(D)PT locks the C- and N- terminals of IL-1RI by forming hydrogen bonds with both terminals to adopt a closed conformation and consequently minimizes the system energy. Conclusions: IL-1RI antagonist K(D)PT effectively treated allergic rhinitis. The molecular mechanism of action indicated that K(D)PT connects the C- and N- terminals of IL-1RI via hydrogen bond formation to establish a stable conformation and consequently minimize the system energy of IL-1RI.
1. Introduction
Allergic rhinitis, which causes sneezing, rhinorrhea, and nasal obstruction, is an immune system disorder mediated by IgE in response to exposure to allergens, such as house dust or pollen. Allergic rhinitis results in complications such as sleep disorders, sinus diseases, and asthma flares. Histologically, allergic rhinitis is characterized by a significant increase in eosinophils in the respiratory mucosa and epithelium. Disrupting the balance between TH1 and TH2 cells results in an increased number of eosinophils.1,2 The TH2 cytokine IL-4 induces IgE production, eosinophilia, and the release of eosinophil cationic protein.1,3,4 H1 anti-histamine agents remain the predominant treatments for allergic rhinitis, for example, cetirizine. The acceptability of these therapeutics is limited by their failure as anti-inflammatory agents.5 Other treatments, such as neural pathway inhibitors and allergen-specific immunotherapies, either possess severe side effects or are longer, complicated therapeutic options.6–10 Targeting a single cytokine, such as tumor necrosis factor-α (TNF-α), is an effective strategy for treating inflammatory diseases (for example, rheumatoid arthritis),11,12 increasing interest in therapies targeting cytokines and chemokines. A challenge in this therapeutic approach is the selection of the appropriate cytokine or chemokine targets because allergic rhinitis can express many cytokines with overlapping functions.13 Many cytokines are induced when IL-1RI is activated because IL-1RI plays a key role in both innate immunity and inflammation. IL-1RI stimulates thymocyte proliferation, accessory growth factor activity for certain T helper cells, and the release of several interleukin cytokines.14–16 Therefore, IL-1RI is closely associated with allergic diseases, including allergic rhinitis, asthma, and skin inflammation.17 Drugs targeting IL-1 or IL-1R are currently clinically available (for example, Anakinra, IL-1 trap, and Pralnacasan). Anakinra is an IL-1 inhibitor used to treat inflammatory diseases.18,19 However, the short half-life of Anakinra limits its acceptability.20IL-1RI consists of three extracellular immunoglobulin domains that host an innate agonist (IL-1β), undergo a conformational change, and recruit an IL-1RI accessory protein (IL-1RAcP) to form an active heterodimer.21–23 The tri-peptide K(D)PT is derived from α-MSH (residues 11–13) by replacing Pro12 with D-Pro, and Val13 with Thr.24–28 K(D)PT is also associated with the IL-1β (residues 193–195)29 and exerts anti-inflammatory effects through IL-1RI.24–28 Dominik Bettenworth and co-workers demonstrated that K(D)PT is effective against intestinal inflammation in a mouse model of chronic enterocolitis.30
We hypothesized that IL-1RI antagonist K(D)PT may exhibit anti-rhinitis activity. To prove this hypothesis, we examined the ability of K(D)PT to antagonize IL-1RI using HEK293/IL-1RI cells and tested the functional activity of K(D)PT in an animal model. MD simulations were performed to determine the binding mode for the K(D)PT/IL-1RI complex. There are two IL-1RI co-crystal structures, IL-1RI with the agonist IL-1β (Complex A) and IL-1RI with an antagonist31 (Complex B). Complex A is structurally open, and Complex B is structurally closed. 50 ns MD simulations were performed on unliganded IL-1RI, the IL-1RI/IL-1β complex, and the IL-1RI/K(D)PT complex to elucidate the different binding mechanisms of Complexes A and B. These differences may enable the rational design of IL-1RI antagonists.
2. Results
2.1. K(D)PT down-regulates the IL-1RI mediated expression of IL-2 and IL-4
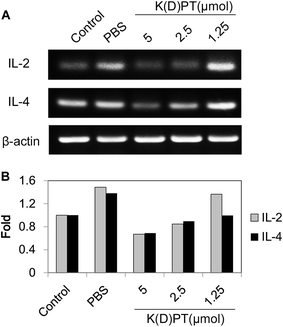
T lymphocytes play a pivotal role in the process of airway hyper-responsiveness, which is the main cause of allergic rhinitis and asthma.32 The role of T lymphocytes involves the production of various inflammatory cytokines and consequently affects downstream signaling.33 Among these cytokines, IL-2 and IL-4 are considered responsible for T cell resistance in airway inflammation.34 Therefore, to elucidate the regulatory effects of K(D)PT on the expression of IL-2 and IL-4, we selected the HEK293/IL-1RI cell line,15 which overexpresses IL-1RI, to perform RT-PCR assays. The mRNA expressions of IL-2 and IL-4 were significantly up-regulated in cells stimulated with 10 ng ml−1 IL-1β (Fig. 1). K(D)PT significantly down-regulated the mRNA expressions of IL-2 and IL-4 in a dose-dependent manner in HEK293/IL-1RI cells. K(D)PT was most effective at 5 μmol; 2.5 μmol K(D)PT had a notable effect, while 1.25 μmol K(D)PT had little effect.2.2. K(D)PT alleviates nasal symptoms of mice with allergic rhinitis
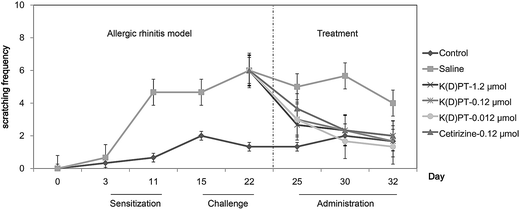
Nose itching, a major symptom of allergic rhinitis,35 results in nose scratching and causes much discomfort, greatly affecting the quality of life of patients with allergic rhinitis.36 To obtain a mouse model of allergic rhinitis, C57BL/6 mice were sensitized to OVA via intraperitoneal OVA injection and then challenged with an intra-nasal dose of OVA. After each treatment, the frequency of nasal scratching was observed for 5 minutes (Fig. 2). Compared with saline-treated mice, the OVA-treated mice exhibited a significant increase in nasal scratching. K(D)PT treatment at different doses significantly decreased nasal scratching, whereas saline had no effect. In addition, the efficacy of K(D)PT was comparable to that of cetirizine, the second generation antihistamine widely used for treating AR.37,38 The effects of K(D)PT were not substantially affected by different doses and this is in agreement with previous reports for this compound.29 This behavior may mean that the interaction of K(D)PT and IL-1RI in vitro is isolated, but the interaction can be influenced by many other cytokines in vivo.2.3. K(D)PT prevents the infiltration of nasal eosinophilia
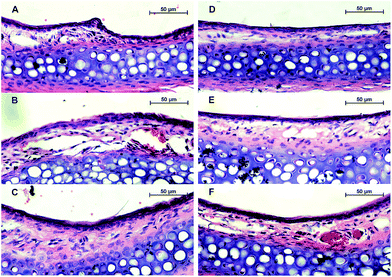
People with allergic rhinitis experience histological changes in their nasal mucosa, such as varying degrees of inflammatory cell infiltration, solid edema, and epithelial damage. In this study, we assessed the distribution and severity of allergen-induced sub-mucosal eosinophilic infiltration. H&E staining indicated that the membrane of nasal mucosa tissue was intact in the control group (Fig. 3). In the mice that were sensitized to OVA, we observed cilia loss and solid edema. In addition, mucosal eosinophil infiltration (bright red staining) was significantly greater in the OVA-sensitized mice than in the control mice. After cetirizine treatment, eosinophil infiltration was significantly alleviated, solid edema was slightly relieved, and cilia loss was recovered. Treatment with 0.12 or 1.2 μmol of K(D)PT improved the morphology of the mucosa tissue by relieving solid edema and decreasing eosinophil infiltration; the low dose of 0.012 μmol of K(D)PT was not sufficient because allergic inflammatory cell infiltration remained, although solid edema was relieved.2.4. K(D)PT down-regulates inflammatory cytokines in mouse serum
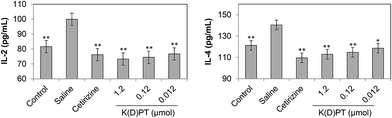
Considering the importance of IL-2 and IL-4 in T cell-mediated inflammation,34 we determined the levels of IL-2 and IL-4 in mouse serum. Serum IL-2 and IL-4 levels were significantly up-regulated in sera from OVA-sensitized mice compared with normal mice (Fig. 4). After treatment with cetirizine or different doses of K(D)PT, IL-2 and IL-4 levels were notably lower, suggesting that K(D)PT inhibits inflammation. These data indicate that IL-1RI is involved in inflammatory diseases.2.5. IL-1RI is highly expressed in animals with allergic rhinitis
Immunohistochemistry was performed to verify IL-1RI expression in the nasal mucosa of mice after various treatments. K(D)PT decreased IL-1RI expression (Fig. 5). IL-1RI was highly expressed in mice with allergic rhinitis (Fig. 5B), indicating that IL-1RI is a target for the treatment of inflammatory disease. After treatment with 0.12 μmol of K(D)PT or cetirizine daily, IL-1RI expression was significantly reduced in the mice. In summary, K(D)PT exerts anti-inflammatory activity by reducing the expression of IL-1RI under inflammatory conditions.2.6. Molecular mechanism of action of K(D)PT as an IL-1RI antagonist
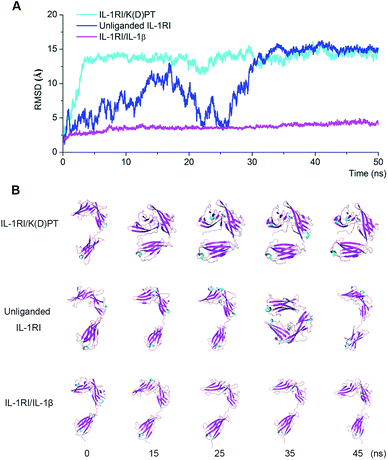
Fifty-ns MD simulations on the three systems (unliganded IL-1RI, the IL-1RI/IL-1β complex, and the IL-1RI/K(D)PT complex) indicated that the backbone RMSD of IL-1RI in the IL-1RI/IL-1β complex did not undergo notable fluctuations during the entire simulation (Fig. 6A). This result is consistent with the snapshots extracted from the MD trajectories. The conformations of the IL-1RI/IL-1β complex did not undergo major changes (Fig. 6B), and the structure remained open.The top-ten docked poses of K(D)PT were chosen as starting points for MD simulations. Ten ns MD simulations demonstrated that one of the ten poses represents stable conformation. Therefore, this pose was used as the starting point to conduct MD simulations. Fifty ns MD simulation indicated: the backbone RMSD of IL-1RI in the IL-1RI/K(D)PT complex underwent significant change at approximately 3 ns and remained relatively stable after 3 ns. The structure of the IL-1RI/K(D)PT complex remained closed (Fig. 6A) during the entire simulation. Initially, IL-1RI formed a pocket (residues 240–264) to host K(D)PT. Hydrogen bonds formed between K(D)PT (Lys and Thr) and IL-1RI (Glu253, Ile244 and Trp237). During the first 5 ns of the simulation, the IL-1RI conformation changed from open to closed (Fig. 6B) and remained closed since then. This closed conformation is consistent with the crystal structure (PDB code: 1 G0Y) reported by Vigers.31 Meanwhile, the K(D)PT conformation underwent minor adjustments to accommodate the groove at the C-terminal of IL-1RI (Fig. 7). Between 5 and 25 ns in the simulation, the K(D)PT conformation significantly changed; the hydrogen bonds between K(D)PT and IL-1RI (at Trp237 and Asp245) were destroyed, and the Lys residue in K(D)PT flipped to form new hydrogen bonds between K(D)PT (at Lys) and IL-1RI (at Ile244, Glu246, and Glu253) at the C-terminal (Fig. 7). Meanwhile, hydrogen bonds formed between the Thr in K(D)PT and Glu2 (at the N-terminal) of IL-1RI (Fig. 7).
The backbone RMSD of unliganded IL-1RI underwent large fluctuations during the entire simulation (blue curve in Fig. 6A). In the absence of ligand, IL-1RI was flexible and capable of recruiting a ligand. The conformation of unliganded IL-1RI randomly switched between open and closed (Fig. 6B).
Principal component analyses (PCA) of the MD trajectories of the IL-1RI/K(D)PT complex and unliganded IL-1RI identified the most significant motions of the IL-1RI/K(D)PT complex and unliganded IL-1RI. The first two principal components of the IL-1RI/K(D)PT complex accounted for 60.1% and 24.4% of the overall motion; the first two principal components of unliganded IL-1RI accounted for 46.7% and 4.2% of the overall motion. For the IL-1RI/K(D)PT complex, the first component (PCA1) consisted of mainly the closing motion (Fig. 8A): domains I and III moved closer to each other. The second component (PCA2) contained several motions (Fig. 8B): domains I and III twisted counter-clockwise toward the same plane. To summarize, K(D)PT induced IL-1RI to adopt a closed state by forming hydrogen bonds with domains I and III.
In the unliganded IL-1RI system, domains I and III were connected to domain II and randomly moved closer or apart (Fig. 8C and D).
Energy landscape maps for the three systems, IL-1RI/K(D)PT (Fig. 9A), unliganded IL-1RI (Fig. 9B), and IL-1RI/IL-1β (Fig. 9C), were generated from the 50 ns MD simulations. The energy landscape map of the IL-1RI/K(D)PT complex revealed declining system energy path, starting with a sharp energy decrease of approximately −5 kcal mol−1 at −25 of PCA1 (equivalent to approximately 25 ns) to approximately −8 kcal mol−1 at 50 of PCA1 (equivalent to approximately 50 ns) (Fig. 9A).
However, the energy landscape map of unliganded IL-1RI indicated that the system energy remained nearly the same, approximately −6 kcal mol−1, during the entire simulation. The system energy evolution path was random (Fig. 9B). The IL-1RI/IL-1β system exhibited similar behavior (Fig. 9C).
The energy landscape analyses further demonstrated that K(D)PT stabilizes the closed conformation of IL-1RI.
3. Experimental
3.1. Ethics statement
This study was performed in strict accordance with the recommendations of the Institutional Animal Care and Use Committee of Sun Yat-Sen University (IACUC, SYSU). All procedures were approved by the Animal Ethical and Welfare Committee of Sun Yat-Sen University. All efforts were made to minimize suffering.3.2. Cell culture and stimulation
HEK293/IL-1RI cells stably expressing IL-1RI15 were a kind gift from Dr X. Li (Cleveland Clinic, OH, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C in 5% CO2. For stimulation, the cells were seeded into 6-well micro-plates. At 80% confluence, the cells were treated with K(D)PT (Jetway, Guangzhou, China) at the indicated concentrations for 2 hours. IL-1β (1 ng ml−1) was then added, and the cells were incubated for an additional 2 hours. The cells were then harvested for further analysis.3.3. Reverse-transcription PCR
Total RNA was isolated using RNAiso Plus (TaKaRa, Dalian, China) according to the manufacturer's protocol. Total RNA (1 μg) was converted to cDNA using Oligo (dT) 18. cDNA was used to amplify specific target genes by PCR. β-Actin was used as the RNA loading control. The PCR products were separated on 1% (w/v) agarose gels and analyzed using an Alpha Imager EP (Alpha Innotech Corp., CA, USA). The following PCR primer sequences were used: β-actin, sense 5′-TGGAATCCTGTGGCATCCATGAAA-3′ and antisense 5′-TAAAACGCAGCTCAGTAACAGTCC-3′; IL-2, sense 5′- TCCAGAACATGCCGCAGAG-3′ and antisense 5′- CCTGAGCAGGATGGAGAATTACA-3′; and IL-4, sense 5′- TCGACACCTATTAATGGGTCTCACC-3′ and antisense 5′- CAAAGTTTTGATGATCTCCTGTAAG-3′.3.4. Animal study
Male 4- to 6 week-old C57BL/6 mice were purchased from the Medical Experimental Animal Center of Guangdong province. All animals were housed under Specific Pathogen Free (SPF) conditions at the Laboratory Animal Center of Sun Yat-Sen University. All animal experiments in this study were approved by the Animal Ethical and Welfare Committee of Sun Yat-Sen University.The allergic rhinitis model was developed according to the method published by Hiroko Saito39 with a few modifications. Briefly, after 3 days of adaptive feeding, the mice were sensitized with an intraperitoneal injection of 10 μg of ovalbumin (OVA, Sigma, St. Louis, USA) in combination with 2 mg of aluminum hydroxide (ALU, Shanghai Chemical Reagent Factory, Shanghai, China) dissolved in saline every second day for a total of 7 injections as a general immunization. The mice in the normal control group were given an intraperitoneal injection of saline on the same schedule. After the general immunization, the mice received daily intra-nasal challenges with 4 μl of a 10% OVA solution in saline (g ml−1) for 10 days (600 μg per day), while the mice in the normal control group received intra-nasal challenges with saline.
As a treatment, the sensitized mice received an intra-nasal dose of cetirizine or K(D)PT at different dosages once a day for 10 days. For the control and negative control, an equivalent amount of saline was administered in the same manner.
3.5. Nasal symptoms
To count the number of nasal scratching incidents, all mice were observed for 5 minutes after challenge in accordance with the method published by Masanori M.403.6. Cytokine assays
Twenty-four hours after the last nasal challenge, blood specimens were collected from the mouse orbit and centrifuged at 2000 rpm for 20 minutes; serum was collected and stored at −70 °C. To measure the concentrations of IL-2 and IL-4 in the mouse serum, commercially available ELISA kits (Dakewe Biotech, Beijing, China) were used in accordance with the manufacturer's instructions. Each sample was measured in triplicate.3.7. Histological examination
To evaluate the infiltration of inflammatory cells into the nasal mucosa, histological examinations were performed according to the method published by Mitsuhiro Okano.41 Briefly, the mice were sacrificed after blood collection, and their heads were removed and fixed in a 10% neutral-buffered formalin solution for two weeks, followed by removal of the nasal mucosa and embedding in paraffin. We then followed the standard procedure for processing biopsy samples with H&E (hematoxylin and eosin) staining. For immunohistochemistry, the samples were deparaffinized and prepared according to standard protocols. IL-1RI was labeled with an IL-1RI antibody (M-20, Santa Cruz, USA) and then detected using a ChemMate™ DAKO Envision™ Detection Kit (DAKO, Glostrup, Tokyo, Denmark) with diaminobenzidine staining. Images were acquired on a microscope (Nikon Eclipse 55i, Japan) equipped with a CCD digital camera (Nikon DS-U3, Tokyo, Japan) and analyzed with dedicated Firmware DS-U2 (Nikon, Tokyo, Japan). Constant condenser and light intensity settings were used throughout the imaging process.3.8. Statistical analysis
The data were analyzed by the unpaired t-test using the SPSS software and are presented as the mean ± SE. P values of less than 0.05 were considered statistically significant.3.9. Molecular dynamics simulations
The extracellular structure of IL-1RI was obtained from the crystal structure (PDB code: 4DEP).23 Missing residues (residues 34–35, 44–47, and 224–225 in the loops of IL-1RI; residues 227–242, 268–275, and 297–309 in the loops of IL-1RAcp) were fixed using the homology modeling module of Molecular Operating Environment 2012.10 (MOE, Chemical Computing Group Inc. Montreal, Canada). To study the impact of K(D)PT on IL-1RI, three systems were constructed. In the first system, K(D)PT was docked into the loop between two β strands (fragment 240–264) located in the third Ig-like domain of IL-1RI using the dock module of MOE.42 The ligand coordinates were taken from docking result. The structure of K(D)PT was subjected to geometric optimization using the HF/6-31G(d) basis set from Gaussian 09 (ref. 43). The second system consisted of IL-1RI only, and the third system contained IL-1RI and its endogenous ligand, IL-1β.GPU-based44,45 MD simulations were performed using the PMEMD module in AMBER 12.46 The partial atomic charges of the ligands were calculated in the Gaussian 09 (ref. 43) program using the Hartree–Fock method with the 6-31G(d) basis set. The antechamber program was then used to fit the restricted electrostatic potential (RESP) and to assign the GAFF force field parameters.47 For the proteins, the AMBER ff12SB force field was used.48,49 The ligand–receptor complexes were neutralized by adding sodium/chlorine counter ions and were solvated in an octahedral box of TIP3P50 water molecules with solvent layers of 10 Å between the box edges and the solute surface. The SHAKE51,52 algorithm was used to restrict all covalent bonds involving hydrogen atoms with a time step of 2 femtoseconds (fs). The Particle-mesh Ewald (PME) method53 was applied to treat long-range electrostatic interactions.
For each ligand–receptor system, three steps of minimization were performed before the heating step. First, all atoms in the receptor-ligand complex were restrained with 50 kcal (mol−1 Å−2), whereas the solvent molecules were not restrained. This step included 2000 cycles of steepest descent minimization and 2000 cycles of conjugated gradient minimization. Second, all heavy atoms were restrained with 10 kcal (mol−1 Å−2) during the minimization steps, which included 2500 cycles of steepest descent minimization and 2500 cycles of conjugated gradient minimization. The third step included 5000 cycles of steepest descent minimization and 5000 cycles of conjugated gradient minimization without restraint.
After the energy minimizations, the whole system was first heated from 0 to 300 K in 50 picoseconds (ps) using Langevin dynamics at a constant volume and then equilibrated for 400 ps at a constant pressure of 1 atm. A weak constraint of 10 kcal (mol−1 Å−2) was used to restrain all heavy atoms in the receptor–ligand complexes during the heating steps. Finally, periodic boundary dynamic simulations were conducted on the whole system with an NPT (constant composition, pressure, and temperature) ensemble at a constant pressure of 1 atm and 300 K in the production step. Each receptor–ligand solution complex was simulated for 50 ns. The coordinates of each system were saved every 10 ps. The root-mean-square deviations (RMSDs) of the original receptors in the complexes were calculated.
3.10. Principle component analysis
Principle component analysis (PCA)54 was utilized to ascertain the collective motions of each system using the positional covariance matrix C of the atomic coordinates and its eigenvectors. The elements of the positional covariance matrix C were defined by eqn (1):| Ci = 〈(xi − 〈xi〉)(xj − 〈xj〉)〉 (i, j = 1, 2, 3,…, 3N) | (1) |
3.11. Energy landscape analysis
The energy landscapes of the proteins during the conformational changes were obtained using an appropriate conformational sampling method. Conformations produced by the MD simulations were used for the energy analysis in this study. A covariance matrix of Cα was generated and used to analyze 3 eigenvectors to obtain modes for each eigenvector. After calculating the 3 eigenvectors, snapshots were then projected onto these eigenvectors in an additional sweep through the trajectory. In a scatter diagram based on the two eigenvectors, the energy decreased as the intensity of the projections increased. Thus, to obtain a three-dimensional (3D) representation of the energy landscape, we divided the “scatter diagram” into N × N gridding and calculated the distribution probability of each grid. The energy landscape was then obtained using eqn (2):55–57
G(x) = −kBT × ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(x) P(x)
| (2) |
4. Conclusion
Using HEK293/IL-1RI cellular assays and animal models, we determined that IL-1RI antagonist K(D)PT exhibits anti-rhinitis activity. At the cellular level, K(D)PT down-regulated the IL-1β-mediated induction of IL-2 and IL-4 mRNA expression. In a mouse model, K(D)PT suppressed the inflammation-induced increase in serum IL-2 and IL-4. In addition, K(D)PT alleviated nose itching, mucosal eosinophil infiltration, and solid edema in mice with allergic rhinitis.Based on the 50 ns MD simulations, the following molecular mechanism of action of K(D)PT as an IL-1RI antagonist can be proposed: (1) in the IL-1RI/IL-1β complex, IL-1RI is in the open conformation; (2) in unliganded IL-1RI, domains I and III randomly move closer or apart without significant energetic changes; (3) K(D)PT induces IL-1RI to adopt a closed conformation by forming hydrogen bonds with domains I and III. The closed conformation significantly reduces the system energy. These findings provide novel avenues for the rational design of IL-1RI antagonists.
Competing interests
The authors declare no competing financial interest.Author's contributions
The main concepts, experiment design, manuscript writing: CJL. Cell experiment: CJL and YLL. Animal experiment: CJL, LJC, GDZ, YR and QH. Molecular dynamics simulation and data analysis: CJL, HG, ZZZ, and BC. Revising and submitting the manuscript: QG and JX.Acknowledgements
This work was funded in part of the National Natural Science Foundation of China (no. 81173470), the National High-tech R&D Program of China (863 Program) (2012AA020307), Guangdong Provincial Key Laboratory of Construction Foundation (2011A060901014), and the Special Funding Program for the National Supercomputer Center in Guangzhou (2012Y2-00048/2013Y2-00045, 201200000037). We thank Dr X. Li (Cleveland Clinic, Cleveland, OH) for generous providing HEK293/IL-1RI cell.References
- M. Benson, I. L. Strannegard, O. Strannegard and G. Wennergren, J. Allergy Clin. Immunol., 2000, 106, 307–312 CrossRef CAS PubMed.
- Q. Hamid and M. Tulic, Annu. Rev. Physiol., 2009, 71, 489–507 CrossRef CAS PubMed.
- S. Romagnani, P. Parronchi, M. M. D'Elios, P. Romagnani, F. Annunziato, M. P. Piccinni, R. Manetti, S. Sampognaro, C. Mavilia, M. De Carli, E. Maggi and G. F. Del Prete, Int. Arch. Allergy Immunol., 1997, 113, 153–156 CrossRef CAS PubMed.
- D. Y. M. Leung, Pediatr. Res., 1997, 42, 559–568 CrossRef CAS PubMed.
- F. M. Baroody and R. M. Naclerio, Allergy, 2000, 55, 17–27 CrossRef.
- R. Uddman, L. Cantera, L. O. Cardell and L. Edvinnsson, Ann. Otol., Rhinol., Laryngol., 1999, 108, 969–973 CAS.
- M. Korsgren, J. S. Erjefalt, J. Hinterholzl, R. Fischer-Colbrie, C. A. Emanuelsson, M. Andersson, C. G. Persson, A. Mackay-Sim, F. Sundler and L. Greiff, Am. J. Respir. Crit. Care Med., 2003, 167, 1504–1508 CrossRef PubMed.
- S. Dunzendorfer, P. Schratzberger, N. Reinisch, C. M. Kahler and C. J. Wiedermann, Blood, 1998, 91, 1527–1532 CAS.
- A. J. Frew, J. Allergy Clin. Immunol., 2003, 111, S712–S719 CrossRef.
- H. S. Nelson, J. Allergy Clin. Immunol., 2003, 111, S793–S798 CrossRef.
- L. B. van de Putte, C. Atkins, M. Malaise, J. Sany, A. S. Russell, P. L. van Riel, L. Settas, J. W. Bijlsma, S. Todesco, M. Dougados, P. Nash, P. Emery, N. Walter, M. Kaul, S. Fischkoff and H. Kupper, Ann. Rheum. Dis., 2004, 63, 508–516 CrossRef CAS PubMed.
- F. C. Breedveld, M. H. Weisman, A. F. Kavanaugh, S. B. Cohen, K. Pavelka, R. van Vollenhoven, J. Sharp, J. L. Perez and G. T. Spencer-Green, Arthritis Rheum., 2006, 54, 26–37 CrossRef CAS PubMed.
- S. T. Holgate and D. Broide, Nat. Rev. Drug Discovery, 2003, 2, 902–914 CrossRef PubMed.
- J. Sims, C. March, D. Cosman, M. Widmer, H. MacDonald, C. McMahan, C. Grubin, J. Wignall, J. Jackson, S. Call and A. Et, Science, 1988, 241, 585–589 CAS.
- Z. Cao, W. J. Henzel and X. Gao, Science, 1996, 271, 1128–1131 CAS.
- E. A. Kurt-Jones, S. Hamberg, J. Ohara, W. E. Paul and A. K. Abbas, J. Exp. Med., 1987, 166, 1774–1787 CrossRef CAS.
- L. J. Rosenwasser, J. Allergy Clin. Immunol., 1998, 102, 344–350 CrossRef CAS.
- A. Courcoul, E. Vignot and R. Chapurlat, Joint Bone Spine, 2014, 81, 175–177 CrossRef PubMed.
- U. Huffmeier, M. Watzold, J. Mohr, M. P. Schon and R. Mossner, Br. J. Dermatol., 2014, 170, 202–204 CrossRef CAS PubMed.
- M. Braddock and A. Quinn, Nat. Rev. Drug Discovery, 2004, 3, 330–339 CrossRef CAS PubMed.
- C. A. Dinarello, Annu. Rev. Immunol., 2009, 27, 519–550 CrossRef CAS PubMed.
- L. A. O'Neill, Immunol. Rev., 2008, 226, 10–18 CrossRef PubMed.
- C. Thomas, J. F. Bazan and K. C. Garcia, Nat. Struct. Mol. Biol., 2012, 19, 455–457 CAS.
- T. Brzoska, T. A. Luger, C. Maaser, C. Abels and M. Bohm, Endocr. Rev., 2008, 29, 581–602 CrossRef CAS PubMed.
- S. Grabbe, R. S. Bhardwaj, K. Mahnke, M. M. Simon, T. Schwarz and T. A. Luger, J. Immunol., 1996, 156, 473–478 CAS.
- T. E. Scholzen, Endocrinology, 2003, 144, 360–370 CrossRef CAS PubMed.
- U. Raap, T. Brzoska, S. Sohl, G. Path, J. Emmel, U. Herz, A. Braun, T. Luger and H. Renz, J. Immunol., 2003, 171, 353–359 CrossRef CAS.
- A. Kokot, A. Sindrilaru, M. Schiller, C. Sunderkotter, C. Kerkhoff, B. Eckes, K. Scharffetter-Kochanek, T. A. Luger and M. Bohm, Arthritis Rheum., 2009, 60, 592–603 CrossRef CAS PubMed.
- A. Mastrofrancesco, A. Kokot, A. Eberle, N. C. J. Gibbons, K. U. Schallreuter, E. Strozyk, M. Picardo, C. C. Zouboulis, T. A. Luger and M. Bohm, J. Immunol., 2010, 185, 1903–1911 CrossRef CAS PubMed.
- D. Bettenworth, M. Buyse, M. Bohm, R. Mennigen, I. Czorniak, K. Kannengiesser, T. Brzoska, T. A. Luger, T. Kucharzik, W. Domschke, C. Maaser and A. Lugering, Am. J. Pathol., 2011, 179, 1230–1242 CrossRef CAS PubMed.
- G. P. Vigers, D. J. Dripps, C. K. Edwards, 3rd and B. J. Brandhuber, J. Biol. Chem., 2000, 275, 36927–36933 CrossRef CAS PubMed.
- D. S. Robinson, Q. Hamid, M. Jacobson, S. Ying, A. B. Kay and S. R. Durham, Springer Semin. Immunopathol., 1993, 15, 17–27 CrossRef CAS.
- T. R. Mosmann and R. L. Coffman, Annu. Rev. Immunol., 1989, 7, 145–173 CrossRef CAS PubMed.
- Q. Liang, L. Guo, S. Gogate, Z. Karim, A. Hanifi, D. Y. Leung, M. M. Gorska and R. Alam, J. Immunol., 2010, 185, 5704–5713 CrossRef CAS PubMed.
- D. P. Skoner, J. Allergy Clin. Immunol., 2001, 108, S2–S8 CrossRef CAS.
- E. O. Meltzer, J. Allergy Clin. Immunol., 2001, 108, S45–S53 CrossRef CAS.
- F. M. Baroody and R. M. Naclerio, Allergy, 2000, 55(suppl. 64), 17–27 CrossRef.
- P. J. Turner and A. S. Kemp, J. Paediatr. Child Health, 2012, 48, 302–310 CrossRef PubMed.
- H. Saito, K. Matsumoto, A. E. Denburg, L. Crawford, R. Ellis, M. D. Inman, R. Sehmi, K. Takatsu, K. I. Matthaei and J. A. Denburg, J. Immunol., 2002, 168, 3017–3023 CrossRef CAS.
- M. Miyata, K. Hatsushika, T. Ando, N. Shimokawa, Y. Ohnuma, R. Katoh, H. Suto, H. Ogawa, K. Masuyama and A. Nakao, Eur. J. Immunol., 2008, 38, 1487–1492 CrossRef CAS PubMed.
- M. Okano, A. R. Satoskar, K. Nishizaki, M. Abe and D. A. Harn, Jr, J. Immunol., 1999, 163, 6712–6717 CAS.
- A. Mastrofrancesco, A. Kokot, A. Eberle, N. C. Gibbons, K. U. Schallreuter, E. Strozyk, M. Picardo, C. C. Zouboulis, T. A. Luger and M. Bohm, J. Immunol., 2010, 185, 1903–1911 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Wallingford, CT, 2009, DOI:citeulike-article-id: 9096580.
- R. Salomon-Ferrer, A. W. Götz, D. Poole, S. Le Grand and R. C. Walker, J. Chem. Theory Comput., 2013, 9, 3878–3888 CrossRef CAS.
- A. W. Gotz, M. J. Williamson, D. Xu, D. Poole, S. Le Grand and R. C. Walker, J. Chem. Theory Comput., 2012, 8, 1542–1555 CrossRef CAS PubMed.
- T. A. D. D. A. Case, T. E. Cheatham, III, C. L. Simmerling, J. Wang, R. E. Duke, R. Luo, R. C. Walker, W. Zhang, K. M. Merz, B. Roberts, S. Hayik, A. Roitberg, G. Seabra, J. Swails, A. W. Goetz, I. Kolossváry, K. F. Wong, F. Paesani, J. Vanicek, R. M. Wolf, J. Liu, X. Wu, S. R. Brozell, T. Steinbrecher, H. Gohlke, Q. Cai, X. Ye, J. Wang, M.-J. Hsieh, G. Cui, D. R. Roe, D. H. Mathews, M. G. Seetin, R. Salomon-Ferrer, C. Sagui, V. Babin, T. Luchko, S. Gusarov, A. Kovalenko, and P. A. Kollman, Amber 12, University of California, San Francisco, 2012 Search PubMed.
- G. Mukherjee, N. Patra, P. Barua and B. Jayaram, J. Comput. Chem., 2011, 32, 893–907 CrossRef CAS PubMed.
- V. Hornak, R. Abel, A. Okur, B. Strockbine, A. Roitberg and C. Simmerling, Proteins, 2006, 65, 712–725 CrossRef CAS PubMed.
- W. D. Cornell, P. Cieplak, C. I. Bayly, I. R. Gould, K. M. Merz, D. M. Ferguson, D. C. Spellmeyer, T. Fox, J. W. Caldwell and P. A. Kollman, J. Am. Chem. Soc., 1995, 117, 5179–5197 CrossRef CAS.
- J. Wang, W. Wang, P. A. Kollman and D. A. Case, J. Mol. Graphics Modell., 2006, 25, 247–260 CrossRef CAS PubMed.
- J.-P. Ryckaert, G. Ciccotti and H. J. C. Berendsen, J. Comput. Phys., 1977, 23, 327–341 CrossRef CAS.
- S. Miyamoto and P. A. Kollman, J. Comput. Chem., 1992, 13, 952–962 CrossRef CAS.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089 CrossRef CAS PubMed.
- A. Amadei, A. B. Linssen and H. J. Berendsen, Proteins, 1993, 17, 412–425 CrossRef CAS PubMed.
- E. Papaleo, P. Mereghetti, P. Fantucci, R. Grandori and L. De Gioia, J. Mol. Graphics Modell., 2009, 27, 889–899 CrossRef CAS PubMed.
- R. Zhou, B. J. Berne and R. Germain, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 14931–14936 CrossRef CAS PubMed.
- A. E. García and K. Y. Sanbonmatsu, Proteins: Struct., Funct., Bioinf., 2001, 42, 345–354 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |