Copper-catalyzed cyanoalkylarylation of activated alkenes with AIBN: a convenient and efficient approach to cyano-containing oxindoles†
Wei Wei‡
,
Jiangwei Wen‡,
Daoshan Yang,
Mengyuan Guo,
Laijin Tian,
Jinmao You and
Hua Wang*
The Key Laboratory of Life-Organic Analysis, Key Laboratory of Pharmaceutical Intermediates and Analysis of Natural Medicine, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, China. E-mail: huawang_qfnu@126.com
First published on 12th September 2014
Abstract
A novel, simple, and cost-effective copper-catalyzed direct cyanoalkylarylation of activated alkenes with AIBN has been developed with cheap K2S2O8 as the oxidant. A series of cyano-containing oxindoles that are otherwise difficult to obtain through previous methods were efficiently synthesized using this protocol.
Transition metal-catalyzed direct oxidative difunctionalization of alkenes is one of the most fascinating and powerful tools for constructing various valuable organic compounds.1 Over the past several years, remarkable progress has been made in this area, and some important and useful synthetic methodologies have been developed. In particular, the direct difunctionalization of alkenes, such as arylcarbonylation,2 azidoarylation,3 arylsulfonylation4 aryltrifluoromethylation,5 arylnitration,6 arylphosphorylation7 alkylarylation,8 hydroxyalkylarylation,9 arylalkoxycarbonylation,10 has recently attracted considerable interests of chemists due to it could offer particularly appealing approaches to access various substituted oxindoles, an important class of heterocycles with unique pharmacological and biological activities.11 Through this strategy, of note, some important functional groups such as carbonyl, phosphoryl, azidyl, trifluoromethyl, hydroxyl, nitro, and ester groups could be introduced into the oxindole framework. Moreover, cyano group as a key structural motif widely exists in many pharmaceuticals, agrochemicals, and materials.12 Also, it can serve as versatile building block for various organic transformations. However, the introduction of cyano species into valuable heterocyclic compounds such as oxindoles via the direct oxidative difunctionalization of alkenes remains an extremely challenging but attractive task in current organic chemistry.13
In 2011, Liu et al. reported an elegant work for palladium-catalyzed oxidative cyanoalkylarylation of alkenes with nitriles leading to cyano-containing oxindoles in the presence of stoichiometric amounts of PhI(OPiv)2/AgF/MgSO4 (eqn (1)).14 Nevertheless, when isobutyronitrile with significant steric effects was used as the substrate, the corresponding cyano-containing product was not obtained even at high temperature (eqn (1)). This well developed method may suffer from some disadvantages of expensive transition metal catalysts, relatively complex reaction conditions, and limited substrate scope, which thereby can limit the applications of this transformation on a large scale. Therefore, there is a great demand for the development of simple, convenient, efficient and alternative strategy to access more diverse cyano-containing oxindoles via direct difunctionalization of alkenes.
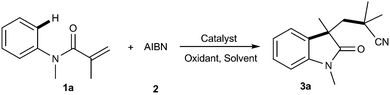
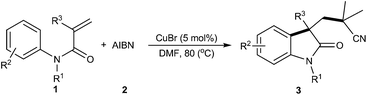
With continuing interests on the development of new methods for difunctionalization of alkenes to obtain important organic compounds,4b,5e,15 herein, we have proposed a novel, convenient, and cost-effective protocol for the construction of cyano-containing oxindoles by copper-catalyzed direct oxidative cyanoalkylarylation of activated alkenes with AIBN, with simple and cheap K2S2O8 as the oxidant (eqn (2)). The present methodology provides a highly attractive and complementary approach to a diverse range of cyano substituted oxindoles in moderate to high yields, together with excellent functional group tolerance through a radical process.
 | (1) |
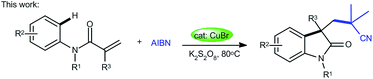 | (2) |
In an initial study, the reaction of N-methyl-N-arylacrylamide 1a with AIBN was investigated by using a variety of transition metal complexes as catalysts, including Pd, Fe, Ag, Cu, Ni, Zn, and In salts, in the presence of K2S2O8 (Table 1 and ESI†). Among the above metal salts examined, Cu salts especially CuBr was found to be the most effective one achieving the desired product 3a in 83% yield (Table 1, entry 6). The structure of 3a was further unambiguously confirmed by single-crystal X-ray analysis (Fig. 1). Further experiments of oxidant screening with CuBr as the catalyst revealed that K2S2O8 was superior to the others such as (NH4)2S2O8, Na2S2O8 TBHP, DTBP, PhI(OAc)2 and H2O2 (Table 1, entries 6–13). The effects of different solvents on this reaction were also examined, and DMF was proved to be better than the others (Table 1, entries 14–20). Among the reaction temperatures were tested, it turned out that the reaction at 80 °C gave the best results (Table 1, entries 6, 21–23). Furthermore, when the reaction was performed in the presence of K2S2O8 or CuBr, the desired product was obtained in 16% and 56% yields, respectively, nevertheless, only a trace amount of desired product 3a was detected when the reaction was performed in the absence of catalyst and oxidant (Table 1, entry 25).
| Entry | Catalyst | Oxidant (1 equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: N-aryl acrylamide 1a (0.25 mmol), AIBN 2 (1 mmol), catalyst (5 mol%), oxidant (2 equiv.), solvent (1 mL), 80 °C, 24 h. n.r. = no reaction. TBHP: tert-butyl hydroperoxide, 70% solution in water; DTBP: Di-tert-butyl peroxide.b Isolated yields based on 1a.c 25 °C.d 60 °C.e 100 °C. | ||||
| 1 | CuI | K2S2O8 | DMF | 66 |
| 2 | Cu(OAc)2 | K2S2O8 | DMF | 80 |
| 3 | Cu(OTf)2 | K2S2O8 | DMF | 63 |
| 4 | CuCl2 | K2S2O8 | DMF | 48 |
| 5 | CuCN | K2S2O8 | DMF | 78 |
| 6 | CuBr | K2S2O8 | DMF | 83 |
| 7 | CuBr | Na2S2O8 | DMF | 46 |
| 8 | CuBr | (NH4)2S2O8 | DMF | 73 |
| 9 | CuBr | TBHP | DMF | 80 |
| 10 | CuBr | DTBP | DMF | 74 |
| 11 | CuBr | Air (O2) | DMF | 56 |
| 12 | CuBr | PhI(OAc)2 | DMF | 72 |
| 13 | CuBr | H2O2 | DMF | 70 |
| 14 | CuBr | K2S2O8 | CH3CN | 47 |
| 15 | CuBr | K2S2O8 | Toluene | 62 |
| 16 | CuBr | K2S2O8 | DME | 58 |
| 17 | CuBr | K2S2O8 | DMSO | 46 |
| 18 | CuBr | K2S2O8 | THF (reflux) | 45 |
| 19 | CuBr | K2S2O8 | 1,4-Dioxane | 33 |
| 20 | CuBr | K2S2O8 | DCE | 77 |
| 21 | CuBr | K2S2O8 | DMF | tracec |
| 22 | CuBr | K2S2O8 | DMF | 55d |
| 23 | CuBr | K2S2O8 | DMF | 75e |
| 24 | — | K2S2O8 | DMF | 16 |
| 25 | — | — | DMF | Trace |
 | ||
| Fig. 1 The crystal structure of 3a. ORTEP drawing of C15H18N2O with 50% probability ellipsoids, showing the atomic numbering scheme. | ||
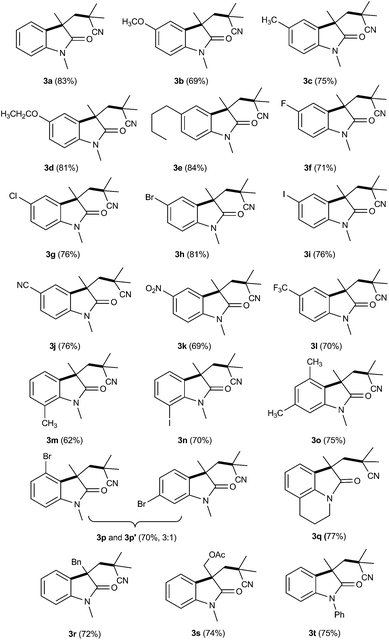
With the optimized conditions in hand, we next examined the reactions of various substituted N-arylacrylamides with AIBN to probe the scope and limitations of the reaction. As shown in Table 2, N-arylacrylamides with electron-donating or electron-deficient substituents at aromatic ring moieties reacted smoothly to afford the desired products in moderate to good yields (3a–3l). Notably, diverse functional groups, including F, Cl, Br, I, cyano, and nitro groups could be tolerated, with corresponding products obtained in good yields (3f–3k). To our delight, the sterically congested ortho substituted substrates were compatible with this reaction to give products 3m and 3n in 62% and 70% yields, respectively. Furthermore, multisubstituted arylacrylamide was also well tolerated in this process, affording the cyano substituted oxindole 3o in 75% yields. Here, meta-substituted substrate offered a mixture of two regioselective products (3p and 3p′). It should be noted that the present catalytic reaction was also successfully applied to tetrahydroquinoline derivative of acrylamide; the corresponding tricyclic oxindole 3q was obtained with good yield. The effects of substituents on alkenes were subsequently evaluated. In addition to methyl group, substrates bearing benzyl and ester protecting groups were well tolerated to this reaction to furnish the corresponding oxindoles (3r and 3s) in good yields. Finally, the examination of different N-protection groups revealed that alkyl and aryl were appropriate for the reaction (2a–2t), in contrast, N-free and acetyl N-arylacrylamide failed to produce the corresponding product. Nevertheless, no desired products were obtained when other nitriles such as 2,2′-azobis(2,4-dimethyl)valeronitrile and 2,2′-azodi(2-methylbutyronitrile) were employed in the present reaction system.
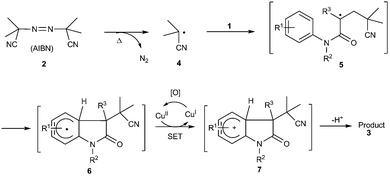
It is well-known that 2-cyanoprop-2-yl radical would be generated from thermal decomposition of AIBN with the release of N2,16 which suggested that the reaction likely proceeded via a single-electron-transfer (SET) process triggered by free 2-cyanoprop-2-yl radical. When 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, a well known radical-capturing species) was added into the present reaction system, the present cyanoalkylarylation reaction was completely suppressed (eqn (3)). Accordingly, a radical pathway should be involved in this transformation.
 | (3) |
Although the mechanism is not completely clear yet, based on the above experimental results and previous reports,2–10,16 a postulated reaction pathway was thereby proposed as shown in Scheme 1. Initially, thermal decomposition of AIBN would lead to the generation of 2-cyanoprop-2-yl radical 4 with the release of N2. Subsequently, the 2-cyanoprop-2-yl radical 4 selectively added to C–C double bond of N-aryl acrylamide 1 giving alkyl radical 5, which underwent an intramolecular radical cyclization reaction leading to intermediate 6. Next, single electron oxidation of intermediate 6 with CuII species to release the cationic intermediate 7. Finally, the hydrogen abstraction of intermediate 7 by K2S2O8 would produce the corresponding cyano-substituted oxindole 3.
In summary, we have successfully employed copper-catalyzed oxidative cyanoalkylarylation of activated alkenes with AIBN for the synthesis of cyano-containing oxindoles. Such a protocol, which utilizes simple and cheap copper salts as catalyst and K2S2O8 as the oxidant, provides a practical, convenient, and efficient approach to various cyano-containing oxindoles. It holds great promise of the potential applications of cyano-containing oxindoles in synthetic and pharmaceutical chemistry. The detailed scope, mechanism, and synthetic application of this reaction are under investigation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21302109, 21302110, and 21375075), the Taishan Scholar Foundation of Shandong Province, the Excellent Middle-Aged and Young Scientist Award Foundation of Shandong Province (BS2013YY019), the Natural Science Foundation of Shandong Province (ZR2013BM007) and the Scientific Research Foundation of Qufu Normal University (BSQD 2012020).References
- Selective reviews see: (a) B. Jacques and K. MuÇiz, in Catalyzed Carbon-Heteroatom Bond Formation, ed. A. K.Yudin, Wiley-VCH, Weinheim, 2010, p. 119 Search PubMed; (b) P. A. Sibbald, Palladium-catalyzed oxidative difunctionalization of alkenes: New reactivity and new mechanisms, ProQuest, UMI Dissertation Publishing, 2011 Search PubMed; (c) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed; (d) A. Minatti and K. MuÇiz, Chem. Soc. Rev., 2007, 36, 1142 RSC; (e) K. H. Jensen and M. S. Sigman, Org. Biomol. Chem., 2008, 6, 4083 RSC; (f) K. MuÇiz, Angew. Chem., Int. Ed., 2009, 48, 9412 CrossRef PubMed.
- (a) M.-B. Zhou, R.-J. Song, X.-H. Ouyang, Y. Liu, W.-T. Wei, G.-B. Deng and J.-H. Li, Chem. Sci., 2013, 4, 2690 RSC; (b) H. Wang, L.-N. Guo and X.-H. Duan, Adv. Synth. Catal., 2013, 355, 2222 CrossRef CAS.
- (a) K. Matcha, R. Narayan and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 7985 CrossRef CAS PubMed; (b) X. H. Wei, Y. Li, A. X. Zhou, T. T. Yang and S. D. Yang, Org. Lett., 2013, 15, 4158 CrossRef CAS PubMed; (c) J. Qiu and R. Zhang, Org. Biomol. Chem., 2014, 12, 4329 RSC.
- (a) X. Li, X. Xu, P. Hu, X. Xiao and C. Zhou, J. Org. Chem., 2013, 78, 7343 CrossRef CAS PubMed; (b) W. Wei, J. Wen, D. Yang, J. Du, J. You and H. Wang, Green Chem., 2014, 16, 2988 RSC; (c) T. Shen, Y. Yuan, S. Song and N. Jiao, Chem. Commun., 2014, 50, 4115 RSC.
- (a) X. Mu, T. Wu, H. Y. Wang, Y. L. Guo and G. S. Liu, J. Am. Chem. Soc., 2012, 134, 878 CrossRef CAS PubMed; (b) H. Egami, R. Shimizu, S. Kawamura and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 4000 CrossRef CAS PubMed; (c) P. Xu, J. Xie, Q. C. Xue, C. D. Pan, Y. X. Cheng and C. J. Zhu, Chem.–Eur. J., 2013, 19, 14039 CrossRef CAS PubMed; (d) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480 CrossRef CAS PubMed; (e) W. Wei, J. Wen, D. Yang, X. Liu, M. Guo, R. Dong and H. Wang, J. Org. Chem., 2014, 79, 4225 CrossRef CAS PubMed.
- (a) Y.-M. Li, X.-H. Wei, X.-A. Li and S.-D. Yang, Chem. Commun., 2013, 49, 11701 RSC; (b) T. Shen, Y. Z. Yuan and N. Jiao, Chem. Commun., 2014, 50, 554 RSC.
- Y.-M. Li, M. Sun, H.-L. Wang, Q.-P. Tia and S.-D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed.
- (a) J. Xie, P. Xu, H. Li, Q. Xue, H. Jin, Y. Cheng and C. Zhu, Chem. Commun., 2013, 49, 5672 RSC; (b) W.-T. Wei, M.-B. Zhou, J.-H. Fan, W. Liu, R.-J. Song, Y. Liu, M. Hu, P. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed.
- L.-N. Guo, H. Wang and X.-H. Duan, Chem. Commun., 2013, 49, 7540 RSC.
- X. Xu, Y. Tang, X. Li, G. Hong, M. Fang and X. Du, J. Org. Chem., 2014, 79, 446 CrossRef CAS PubMed.
- For some selected reviews, see: (a) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36 CrossRef CAS; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (c) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247 RSC; (d) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed; (e) K. Shen, X. Liu, L. Lin and X. Feng, Chem. Sci., 2012, 3, 327 RSC; (f) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023 CrossRef CAS.
- (a) A. J. Fatiadi, in Preparation and Synthetic Applications of Cyano Compounds, ed. S.Patai and Z.Rappaport, Wiley, New York, 1983 Search PubMed; (b) R. C. Larock, Comprehensive Organic Transformations, VCH, New York, 1989 Search PubMed.
- (a) A. Pinto, Y. Jia, L. Neuville and J. Zhu, Chem.–Eur. J., 2007, 13, 961 CrossRef CAS PubMed; (b) Y. Yasui, H. Kamisaki and Y. Takemoto, Org. Lett., 2008, 10, 3303 CrossRef CAS PubMed; (c) Y. Yasui, H. Kamisaki, T. Ishida and Y. Takemoto, Tetrahedron, 2010, 66, 1980 CrossRef CAS PubMed; (d) H. Zhang, P. Chen and G. Liu, Synlett, 2012, 23, 2749 CrossRef CAS PubMed; (e) Y. Wu and G. Liu, Tetrahedron Lett., 2011, 52, 6450 CrossRef CAS PubMed.
- T. Wu, X. Mu and G. Liu, Angew. Chem., Int. Ed., 2011, 50, 12578 CrossRef CAS PubMed.
- W. Wei, C. Liu, Da. Yang, J. Wen, J. You, Y. Suo and H. Wang, Chem. Commun., 2013, 49, 10239 RSC.
- (a) R. Spaccini, N. Pastori, A. Clerici, C. Punta and O. Porta, J. Am. Chem. Soc., 2008, 130, 18018 CrossRef CAS PubMed; (b) M. N. C. Balili and T. Pintauer, Inorg. Chem., 2009, 48, 9018 CrossRef CAS PubMed; (c) M. N. C. Balili and T. Pintauer, Inorg. Chem., 2010, 49, 5642 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. CCDC 1002316. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra09022e |
| ‡ Authors with equal contribution. |
| This journal is © The Royal Society of Chemistry 2014 |