Simple approach for the immobilization of horseradish peroxidase on poly-l-histidine modified reduced graphene oxide for amperometric determination of dopamine and H2O2†
Abstract
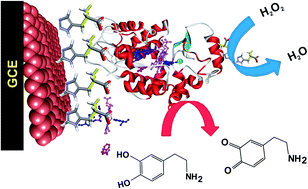
In this work, immobilization of horseradish peroxidase (HRP) on poly-L-histidine (P-L-His) modified reduced graphene oxide (RGO) was demonstrated. The HRP/P-L-His-RGO bio-nanocomposite film was prepared through layer-by-layer (LBL) assembly. Scanning electron microscopy, Fourier transform infrared spectroscopy, electrochemical impedance spectroscopy, and UV-Vis spectroscopy were adopted to monitor the uniformity of the LBL assembly of the HRP/P-L-His-RGO bio-nanocomposite film. The immobilized HRP exhibited excellent electrocatalytic activity towards the reduction of hydrogen peroxide (H2O2). The catalysis currents showed a linear relationship with H2O2 concentration, ranging from 0.2 to 5000 μM. The detection limit (S/N = 3) of H2O2 was 0.05 μM. The apparent Michaelis–Menten constant (Km) was calculated to be 1.2 mM. Moreover, the modified electrode displayed a rapid response (5 s) to H2O2 with good stability and reproducibility. Based on the HRP/P-L-His-RGO bio-nanocomposite film, a third-generation reagentless biosensor was constructed for the determination of H2O2.

 Please wait while we load your content...
Please wait while we load your content...