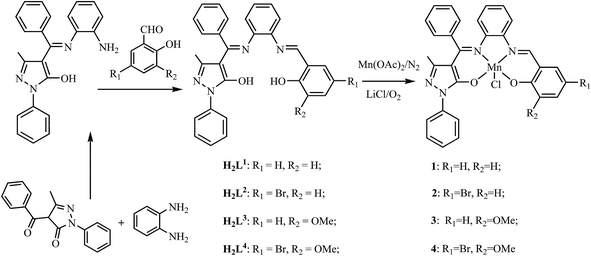
Ring-opening copolymerization of epoxides and anhydrides using manganese(III) asymmetrical Schiff base complexes as catalysts†
Deng-Feng Liu*ab,
Lu-Qun Zhua,
Jing Wua,
Li-Ying Wua and
Xing-Qiang Lü*a
aSchool of Chemical Engineering, Northwest University, Xi'an 710069, Shaanxi, PR China. E-mail: lvxing46@126.com; Tel: +86-29-88302312(O)
bCollege of Chemistry and Chemical Engineering, Xi'an University of Science and Technology, Xi'an 710054, Shaanxi, PR China. E-mail: liudf78@xust.edu.cn; Tel: +86-29-88302312(O)
First published on 27th November 2014
Abstract
Based on a series of asymmetrical Schiff base H2Ln (n = 1–4) ligands with different electronic and steric effects, a series of [Mn(Ln)Cl] complexes 1–4 are obtained and shown to be effective catalysts in cyclohexene oxide and maleic anhydride, cyclohexene oxide and phthalic anhydride, styrene oxide and maleic anhydride or styrene oxide and phthalic anhydride ring-opening copolymerizations. Through the structure design, the input of the electron-withdrawing Br substituent para to the phenoxide group of the complexes is quite beneficial to the improved activities. Moreover, both steric and electronic effects of a suitable MeO substituent at the ortho position of the phenoxide group have much more influence on the formation of alternating ring-opening copolymers than that of selected reaction conditions.
Introduction
Polyesters have found widespread use in drug delivery vesicles, bone screws, scaffolding and suture wires1 due to their good biodegradability and biocompatibility. While these polyesters are typically synthesized by polycondensation reactions from a diol and diacid or the ring-opening polymerization reaction of cyclic esters,2–4 the former route commonly requires high energy input and long reaction time, and inevitably additional side reactions often afford low-molecular-weight polymers, while the latter route is generally limited to the availability of structurally diverse monomers. Taking into account the fact that carbonate segments in polyesters give lower hydrolytic and autocatalytic degradation rates,5 the chain-growth process by copolymerization of carbon dioxide and epoxide6,7 or terpolymerization of carbon dioxide, epoxide and anhydride8–11 has been used to obtain polycarbonates. However, from the viewpoint of mild reaction conditions, the competitive way of copolymerizing an epoxide and anhydride has not been researched extensively in order to obtain diverse polyesters.The catalytic coupling of an epoxide and anhydride with Al(III)–porphyrinato catalysts was previously reported by Inoue12,13 in the 1980s, but the difficulty of obtaining high molecular weight alternating polyesters and the undesirable side reactions of epoxide homopolymerization have impeded its popularity for quite some time, although in the following two decades a series of inorganic metal catalysts (such as Mg(OEt)2 (ref. 14) or Zn3[Co(CN)6]2 (ref. 15)) were used for ring-opening copolymerizations of anhydrides and epoxides. In 2007, Coates et al. reported the obtainment of high molecular weight (up to 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) perfectly alternating polyesters from the solution copolymerization of an alicyclic epoxide and anhydride using just a zinc 2-cyano-β-diketiminato complex as the catalyst,16 which renewed the attention of the scientific area despite of the lower epoxide conversions and catalytic activities for unsaturated anhydride–epoxide copolymerizations. Recently, a series of metal–Porphyrinato,17,18 metal–Salen19–21 and manganese–Corrole complexes22 have been used to catalyze the ring-opening copolymerization of epoxide and anhydride, and a nucleophilic co-catalyst should be assisted to obtain higher monomer conversions and ester contents of the poly(ester-co-ether)s. Especially for the metal–Salen catalyst systems, although the effects of metal active centers, ligand–dimine backbones, axial anionic types or polymerization conditions on the catalytic activity and selectivity of copolymerization from different epoxides and anhydrides have been studied in detail, the catalysts are based on symmetrical Salen-type ligands. To the best of our knowledge, no report on the copolymerization of epoxide and anhydride using the metal complexes based on asymmetrical Salen-type ligands as catalysts have been done. As a matter of fact, the distinctive advantage of asymmetrical Salen-type scaffolds23 or analogies24–26 endows the possibility of further fine-tuning their structures by introducing different electronic and steric effects. Based on different electronic and steric effects, positive impact on the metal active center is produced and the improved catalytic activity and selectivity on the copolymerization of epoxide and anhydride is expected. In addition, manganese complexes were found to be less active compared to chromium or cobalt counterparts described in the literature. Herein, in the search to obtain maximum catalytic performances of manganese complexes for epoxide–anhydride copolymerization, based on a series of new asymmetrical Schiff base ligands H2Ln (n = 1–4) synthesized from the reaction of o-phenylenediamine, 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone (PMBP), and different aldehyde derivatives by the two-step sequence, a series of new catalysts [Mn(Ln)Cl] (n = 1, 1; n = 2, 2; n = 3, 3; n = 4, 4) are obtained. Their ring-opening copolymerization behaviors of familiar epoxide (cyclohexene oxide (CHO) or styrene oxide (SO)) and common anhydride (maleic anhydride (MA) or phthalic anhydride (PA)) with different ring strain in presence of different co-catalysts (DMAP, Ph3P or PPN+Cl−) are examined.
000 g mol−1) perfectly alternating polyesters from the solution copolymerization of an alicyclic epoxide and anhydride using just a zinc 2-cyano-β-diketiminato complex as the catalyst,16 which renewed the attention of the scientific area despite of the lower epoxide conversions and catalytic activities for unsaturated anhydride–epoxide copolymerizations. Recently, a series of metal–Porphyrinato,17,18 metal–Salen19–21 and manganese–Corrole complexes22 have been used to catalyze the ring-opening copolymerization of epoxide and anhydride, and a nucleophilic co-catalyst should be assisted to obtain higher monomer conversions and ester contents of the poly(ester-co-ether)s. Especially for the metal–Salen catalyst systems, although the effects of metal active centers, ligand–dimine backbones, axial anionic types or polymerization conditions on the catalytic activity and selectivity of copolymerization from different epoxides and anhydrides have been studied in detail, the catalysts are based on symmetrical Salen-type ligands. To the best of our knowledge, no report on the copolymerization of epoxide and anhydride using the metal complexes based on asymmetrical Salen-type ligands as catalysts have been done. As a matter of fact, the distinctive advantage of asymmetrical Salen-type scaffolds23 or analogies24–26 endows the possibility of further fine-tuning their structures by introducing different electronic and steric effects. Based on different electronic and steric effects, positive impact on the metal active center is produced and the improved catalytic activity and selectivity on the copolymerization of epoxide and anhydride is expected. In addition, manganese complexes were found to be less active compared to chromium or cobalt counterparts described in the literature. Herein, in the search to obtain maximum catalytic performances of manganese complexes for epoxide–anhydride copolymerization, based on a series of new asymmetrical Schiff base ligands H2Ln (n = 1–4) synthesized from the reaction of o-phenylenediamine, 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone (PMBP), and different aldehyde derivatives by the two-step sequence, a series of new catalysts [Mn(Ln)Cl] (n = 1, 1; n = 2, 2; n = 3, 3; n = 4, 4) are obtained. Their ring-opening copolymerization behaviors of familiar epoxide (cyclohexene oxide (CHO) or styrene oxide (SO)) and common anhydride (maleic anhydride (MA) or phthalic anhydride (PA)) with different ring strain in presence of different co-catalysts (DMAP, Ph3P or PPN+Cl−) are examined.
Experimental
Catalysts preparation
As shown in Scheme 1, reaction of PMBP with o-phenylenediamine in either 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in absolute EtOH gives the sole product of HL0 in ca. 71% yield. When the monoamine precursor HL0 was allowed to react with one of aldehyde derivatives (salicylaldehyde, 5-bromo-salicylaldehyde, o-vanillin or 5-bromo-2-hydroxy-3-methoxy-benzaldehyde) in 1
1 molar ratio in absolute EtOH gives the sole product of HL0 in ca. 71% yield. When the monoamine precursor HL0 was allowed to react with one of aldehyde derivatives (salicylaldehyde, 5-bromo-salicylaldehyde, o-vanillin or 5-bromo-2-hydroxy-3-methoxy-benzaldehyde) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, a series of new ligands H2Ln (n = 1–4) were obtained in good yields, respectively. Furthermore, reaction of equimolar amounts of the ligands H2Ln (n = 1–4) and Mn(OAc)2 under the dry N2 atmosphere and then in the presence of air at room temperature, excess LiCl was added to the solution, resulted in the formation of catalysts 1–4, respectively.
1 molar ratio, a series of new ligands H2Ln (n = 1–4) were obtained in good yields, respectively. Furthermore, reaction of equimolar amounts of the ligands H2Ln (n = 1–4) and Mn(OAc)2 under the dry N2 atmosphere and then in the presence of air at room temperature, excess LiCl was added to the solution, resulted in the formation of catalysts 1–4, respectively.
Catalysts characterization
The four new ligands H2Ln and their complexes 1–4 were well characterized by EA, FT-IR, ESI-MS, 1H NMR and 13C NMR analysis. The solid structure of complex [Mn(L1)Cl(EtOH)] ([1(EtOH)]) as the representative of complexes 1–4 was determined by X-ray single-crystal diffraction analysis27,28 and shown in Fig. 1.Copolymerization procedures of epoxide and anhydride
The copolymerization between epoxide (CHO or SO) and anhydride (MA or PA) were performed in both bulk and solution (toluene) with a stipulated molar ratio of epoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anhydride
anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (co-catalyst) for the selected reaction time.
(co-catalyst) for the selected reaction time.
Results and discussion
Effect of catalyst structure of complexes 1–4 on catalytic copolymerization of CHO and MA
As shown in Scheme 2, the blank experiments in both bulk and solution without catalyst and co-catalyst (entries 1–2 in Table 1) show no copolymers obtained. Simultaneously, co-catalyst DMAP alone (entries 3–4 in Table 1) or catalyst 4 alone (entries 5–6 in Table 1) is not very active (CHO conversions 7–12%), where in solution hardly any activity can be observed and the low molecular weight copolymers (Mn = 500–900 g mol−1) obtained in bulk contain a high percentage of ether linkages (57–65%), which is similar to the results by DMAP alone, Al/Cr–Porphyrinato17,18 or Cr–Salen-catalyzed19–21 ring-opening bulk or solution polymerizations of epoxide and anhydride. In contrast, the incorporation of one of complexes 1–4 as catalyst and DMAP, PPN+Cl− or PPh3 as co-catalyst (other entries in Tables 1–3) not only accelerates the copolymerization, but also strongly stimulates the formation of ester linkages. To study the effect of the electronic and steric environment of the series of asymmetrical Schiff-base complexes 1–4 on the catalytic performance, the copolymerization (entries 7–10 in Table 1) of CHO and MA are carried out in bulk with DMAP as co-catalyst under the stipulated molar ratio (250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of CHO
1) of CHO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA
MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) co-catalyst at polymerization temperature of 110 °C for 150 min. As expected, catalyst 1 gives far higher activity of CHO (87% of CHO conversion) and better selectivity. Interestingly, utilization of the electron-withdrawing Br substituent para to the phenoxide group in catalyst 2, gives relatively higher reactivity (96% of CHO conversion) while lower selectivity, which should be resulted from the increase of electrophilicity of Mn3+ ion in 2 due to the delocalization of electron conjugation. Conversely, the electron-donating MeO substituent ortho to the phenoxide group in catalyst 3 has lower catalytic activity (83% of CHO conversion) and higher selectivity. As to catalyst 4, both the steric effect and electronic effect determine higher catalytic activity (92% of CHO conversion) and selectivity.
co-catalyst at polymerization temperature of 110 °C for 150 min. As expected, catalyst 1 gives far higher activity of CHO (87% of CHO conversion) and better selectivity. Interestingly, utilization of the electron-withdrawing Br substituent para to the phenoxide group in catalyst 2, gives relatively higher reactivity (96% of CHO conversion) while lower selectivity, which should be resulted from the increase of electrophilicity of Mn3+ ion in 2 due to the delocalization of electron conjugation. Conversely, the electron-donating MeO substituent ortho to the phenoxide group in catalyst 3 has lower catalytic activity (83% of CHO conversion) and higher selectivity. As to catalyst 4, both the steric effect and electronic effect determine higher catalytic activity (92% of CHO conversion) and selectivity.
 | ||
| Scheme 2 Synthesis of polyesters from epoxide (CHO or SO) and anhydride (MA or PA) using [Mn(Ln)Cl] (n = 1–4, 1–4) as the catalysts. | ||
| Entry | Method | Cat | Co-cat | Molar ratio | Conv.a (%) | Ethera (%) | Mnb | PDIb |
|---|---|---|---|---|---|---|---|---|
M1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M2 M2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cat Cat![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co-cat Co-cat |
||||||||
| a Determined by 1H NMR.b Determined by GPC. | ||||||||
| 1 | Bulk | — | — | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
— | — | — | — |
| 2 | Solution | — | — | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
— | — | — | — |
| 3 | Bulk | — | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 57 | 900 | 1.12 |
| 4 | Solution | — | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | — | — | — |
| 5 | Bulk | 4 | — | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
7 | 65 | 500 | 1.27 |
| 6 | Solution | 4 | — | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
2 | — | — | — |
| 7 | Bulk | 1 | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 | 5 | 7700 | 1.16 |
| 8 | Bulk | 2 | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 11 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.28 |
| 9 | Bulk | 3 | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | <1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.12 |
| 10 | Bulk | 4 | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 | <1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.09 |
| Entry | Method | Co-cat | Molar ratio | Time min | T °C | Conv.a (%) | Ethera (%) | Mnb | PDIb |
|---|---|---|---|---|---|---|---|---|---|
M1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M2 M2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cat Cat![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co-cat Co-cat |
|||||||||
| a Determined by 1H NMR.b Determined by GPC. | |||||||||
| 1 | Solution | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 110 | 74 | <1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.03 |
| 2 | Bulk | PPh3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 110 | 67 | 6 | 6700 | 1.29 |
| 3 | Bulk | PPNCl | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 110 | 70 | 5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.24 |
| 4 | Bulk | DMAP | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 110 | 98 | 17 | 5400 | 1.33 |
| 5 | Bulk | DMAP | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 110 | 72 | <1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.12 |
| 6 | Bulk | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 90 | 75 | 19 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.68 |
| 7 | Bulk | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 70 | 54 | 8 | 7600 | 1.57 |
| 8 | Bulk | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
300 | 110 | 94 | 13 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.22 |
| 9 | Bulk | DMAP | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 110 | 69 | 4 | 8000 | 1.37 |
Moreover, the structure of catalysts 1–4 also has an important influence on the catalytic selectivity. The CHO–MA copolymer molecular weight (Mn) seems to be related to the reactivity, copolymer obtained from the most active catalyst 2 shows lower Mn value 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 and the high ether content (11%) of the poly(ester-co-ether). The low polymer molecular weight should be attributed to the presence of small amounts of water or hydrolyzed MA that can function as chain transfer agent lowering the polymer molecular weight especially in higher CHO conversion systems,29–33 and higher ether content should be resulted from the heavier side reaction of epoxide homopolymerization.17–21 As to catalysts 3–4, the encumbering substituent (MeO in 3 and 4) ortho to the phenoxide group results in the relatively higher polymer molecular weights (15
900 g mol−1 and the high ether content (11%) of the poly(ester-co-ether). The low polymer molecular weight should be attributed to the presence of small amounts of water or hydrolyzed MA that can function as chain transfer agent lowering the polymer molecular weight especially in higher CHO conversion systems,29–33 and higher ether content should be resulted from the heavier side reaction of epoxide homopolymerization.17–21 As to catalysts 3–4, the encumbering substituent (MeO in 3 and 4) ortho to the phenoxide group results in the relatively higher polymer molecular weights (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–15
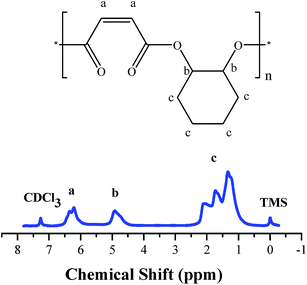
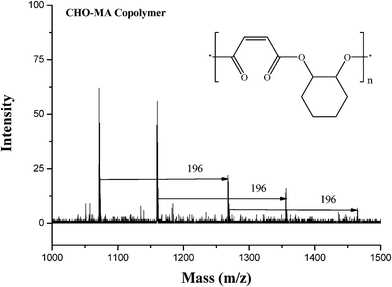
100–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1), which can be explained that the steric effects can effectively suppress polyether formation and increase the molecular weights of polyester, while decreasing the conversion of CHO to a certain extent. The polymer produced by 4/DMAP appears to be almost perfectly alternating CHO–MA copolymer (<1% of the ether content shown in Fig. 2), which is in agreement with the MALDI-TOF-MS spectrum (entry 10 in Table 1) of the product, where an m/z interval of 196 between the consecutive peaks corresponding to the addition of a repeating unit consisting of a CHO plus a MA, thus indicating a perfect alternating microstructure (Fig. 3). The end groups of the distribution are in agreement with a DMAP end-capped, alternating polymer consisting of n[CHO + MA] units.
600 g mol−1), which can be explained that the steric effects can effectively suppress polyether formation and increase the molecular weights of polyester, while decreasing the conversion of CHO to a certain extent. The polymer produced by 4/DMAP appears to be almost perfectly alternating CHO–MA copolymer (<1% of the ether content shown in Fig. 2), which is in agreement with the MALDI-TOF-MS spectrum (entry 10 in Table 1) of the product, where an m/z interval of 196 between the consecutive peaks corresponding to the addition of a repeating unit consisting of a CHO plus a MA, thus indicating a perfect alternating microstructure (Fig. 3). The end groups of the distribution are in agreement with a DMAP end-capped, alternating polymer consisting of n[CHO + MA] units.
Effect of copolymerization procedures of catalyst 4 on catalytic copolymerization of CHO and MA
Catalyst 4 with higher catalytic activity was chosen for further studies in detail with different copolymerization procedures (shown in Table 2). The typical solution polymerization (entry 1 in Table 2) from 4/DMAP under the stipulated molar ratio (250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of CHO
1) of CHO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA
MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) co-catalyst at polymerization temperature of 110 °C for 300 min also produces the expected perfectly alternating CHO–MA copolymer, while it leads to both lower CHO conversion (74%) and higher polymer Mn (18
co-catalyst at polymerization temperature of 110 °C for 300 min also produces the expected perfectly alternating CHO–MA copolymer, while it leads to both lower CHO conversion (74%) and higher polymer Mn (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, PDI = 1.03) than those in bulk as the general feature,19 the higher effective collision frequency of two different monomers may play an important role in solution copolymerization. It is evident that asymmetrical catalyst 4 is better than symmetrical catalyst described in the literature for CHO–MA copolymerization (Mn = 6600–8500 g mol−1, PDI = 2.3–3.5).11,34 To study the effect of co-catalysts on catalytic behaviors, two other types of co-catalysts including Ph3P and PPN+Cl− are compared in combination with catalyst 4 for the bulk copolymerization (entries 2–3 in Table 2). Compared with the effective catalysis of DMAP, Ph3P shows a relatively lower activity (67% of CHO conversion, Mn = 6700 g mol−1 and PDI = 1.29), while the obtained polymer is also the low ether content (6%) poly(ester-co-ether), which can be attributed to the large cone angle of three steric phenyl substituents in PPh3 considerably lowering its nucleophilicity.20 As to PPN+Cl−, it has shown a much higher activity than PPh3 for CHO–MA copolymerization (70% of CHO conversion, Mn = 12
200 g mol−1, PDI = 1.03) than those in bulk as the general feature,19 the higher effective collision frequency of two different monomers may play an important role in solution copolymerization. It is evident that asymmetrical catalyst 4 is better than symmetrical catalyst described in the literature for CHO–MA copolymerization (Mn = 6600–8500 g mol−1, PDI = 2.3–3.5).11,34 To study the effect of co-catalysts on catalytic behaviors, two other types of co-catalysts including Ph3P and PPN+Cl− are compared in combination with catalyst 4 for the bulk copolymerization (entries 2–3 in Table 2). Compared with the effective catalysis of DMAP, Ph3P shows a relatively lower activity (67% of CHO conversion, Mn = 6700 g mol−1 and PDI = 1.29), while the obtained polymer is also the low ether content (6%) poly(ester-co-ether), which can be attributed to the large cone angle of three steric phenyl substituents in PPh3 considerably lowering its nucleophilicity.20 As to PPN+Cl−, it has shown a much higher activity than PPh3 for CHO–MA copolymerization (70% of CHO conversion, Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 and PDI = 1.24).35 In line with the common feature of variation of catalyst and co-catalyst concentration on the catalytic activity,17,18 for DMAP, there is a slight increase in reactivity with increasing catalyst and co-catalyst concentration (entries 4–5 in Table 2), while the polymer molecular weight (5400 g mol−1) decreases with another origin from the chain transfer effect of excess DMAP.20 Considering the effect of the reaction temperature on the polymerization behavior (entries 6–7 in Table 2), lower temperature (70 or 90 °C) results in the lower polymerization rate (54–75% of CHO conversions), the lower polymer molecular weight (Mn = 7600–10
300 g mol−1 and PDI = 1.24).35 In line with the common feature of variation of catalyst and co-catalyst concentration on the catalytic activity,17,18 for DMAP, there is a slight increase in reactivity with increasing catalyst and co-catalyst concentration (entries 4–5 in Table 2), while the polymer molecular weight (5400 g mol−1) decreases with another origin from the chain transfer effect of excess DMAP.20 Considering the effect of the reaction temperature on the polymerization behavior (entries 6–7 in Table 2), lower temperature (70 or 90 °C) results in the lower polymerization rate (54–75% of CHO conversions), the lower polymer molecular weight (Mn = 7600–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1), the broadening PDI (1.57–1.68) and the higher ether content (8–19%) of poly(ester-co-ether), it indicates that a lower reaction temperature shows negative influence on the reaction rate constant under the consistent conditions. According to Arrhenius equation, an elevated temperature favors most of the reactions to a certain degree because their activation energy is usually positive. It appears that higher temperature is more desirable for ring-opening copolymerization of CHO–MA. The effect of polymerization time on the catalytic activity is similar to that of catalyst and co-catalyst concentration, the catalytic activity increases at first from 60 min to 150 min, and then decreases from 150 min to 300 min, and the peak appeared at 150 min. The relatively lower polymer molecular weight (Mn = 11
800 g mol−1), the broadening PDI (1.57–1.68) and the higher ether content (8–19%) of poly(ester-co-ether), it indicates that a lower reaction temperature shows negative influence on the reaction rate constant under the consistent conditions. According to Arrhenius equation, an elevated temperature favors most of the reactions to a certain degree because their activation energy is usually positive. It appears that higher temperature is more desirable for ring-opening copolymerization of CHO–MA. The effect of polymerization time on the catalytic activity is similar to that of catalyst and co-catalyst concentration, the catalytic activity increases at first from 60 min to 150 min, and then decreases from 150 min to 300 min, and the peak appeared at 150 min. The relatively lower polymer molecular weight (Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1) and the larger PDI (1.22) than those (Mn = 15
400 g mol−1) and the larger PDI (1.22) than those (Mn = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 and PDI = 1.09) at the reaction time of 150 min from the same 4/DMAP system, together with the higher ether content (13%) of poly(ester-co-ether) are observed from the longest reaction time of 300 min, it should also be due to the chain transfer effect of DMAP with the longest reaction time.20
600 g mol−1 and PDI = 1.09) at the reaction time of 150 min from the same 4/DMAP system, together with the higher ether content (13%) of poly(ester-co-ether) are observed from the longest reaction time of 300 min, it should also be due to the chain transfer effect of DMAP with the longest reaction time.20
Catalytic copolymerization of other epoxide and anhydride by 4/DMAP
As a matter of fact, as shown in entries 1–3 of Table 3, 4/DMAP system also effectively catalyzes the bulk ring-opening copolymerization of other epoxides and anhydrides under the similar reaction conditions. The decreased ring strain of the copolymerization monomer backbones leads to relatively lower activities and lower polymer molecular weight products,19,36–38 CHO conversion of 84% and Mn = 2400 g mol−1 for CHO–PA copolymerization, SO conversion of 73% and Mn = 5900 g mol−1 for SO–MA copolymerization and SO conversion of 68% and Mn = 2400 g mol−1 for SO–PA. All the copolymers exhibit narrow molecular weight distributions (PDI = 1.08–1.24), and the low ether content poly(ester-co-ether)s are also observed. Moreover, the CHO–PA, SO–MA polymers, especially the SO–PA polymer produced by 4/DMAP, also seem to be perfectly alternating copolymers, which can be further proved by their MALDI-TOF-MS spectra of the products, where an m/z interval of 246, 218 or 268 between the consecutive peaks corresponding to the addition of a [CHO + PA], [SO + MA] or [SO + PA] repeating unit, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of epoxide
1) of epoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anhydride
anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) co-catalyst at polymerization temperature of 110 °C for 150 min
co-catalyst at polymerization temperature of 110 °C for 150 min
Conclusions
In conclusion, a series of asymmetrical Schiff-base catalysts 1–4 are shown to be effective catalysts in CHO–MA, CHO–PA, SO–MA and SO–PA ring-opening copolymerization. Especially for CHO–MA ring-opening copolymerization, catalysts 3–4 are obvious better than symmetrical catalysts described in the literature due to introducing MeO substituent at the ortho orientation on the phenoxide group. In the structure design of catalysts 2–4, the input of electron-withdrawing Br substituents para to the phenoxide group of the complexes is considerately beneficial to their improved activities. Moreover, both steric and electronic effects of suitable MeO substituent at the ortho orientation on the phenoxide group of the complexes 3–4 have much more importance on the formation of perfectly alternating ring-opening copolymers than that of selected reaction conditions. Worth mentioning is the fact, of three co-catalysts tested, moderate DMAP exhibits positive to monomer conversion and chain growth of polymers. Further studies focused on increasing the efficiency and expanding the monomer scope with the asymmetrical Schiff base metal catalysts, are currently in progress.Acknowledgements
This work is funded by the National Natural Science Foundation (21373160), the Scientific Research Program Foundation of Shaanxi Provincial Education Department (2013JK0701) and the Scientific Research Culture Foundation of xi'an University of Science and Technology (201117).References
- A. E. Felber, M. H. Dufresne and J. C. Leroux, Adv. Drug Delivery Rev., 2012, 64, 979–992 CrossRef CAS PubMed.
- M. J.-L. Tschan, E. Brulé, P. Haquette and C. M. Thomas, Polym. Chem., 2012, 3, 836–851 RSC.
- C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC.
- N. Ajellal, J. F. Carpentier, C. Guillaume, S. M. Guillaume, M. Helou, V. Poirier, Y. Sarazin and A. Trifonov, Dalton Trans., 2010, 39, 8363–8376 RSC.
- J. Xu, Z. Liu and R. Zhou, J. Appl. Polym. Sci., 2006, 101, 1988–1994 CrossRef CAS.
- E. Brule, J. Guo, G. W. Coates and C. M. Thomas, Macromol. Rapid Commun., 2011, 32, 169–185 CrossRef CAS PubMed.
- X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462–1484 RSC.
- T. M. K. Tsurutat and S. Inoue, Makromol. Chem., 1964, 75, 211–214 CrossRef.
- R. C. Jeske, J. M. Rowley and G. W. Coates, Angew. Chem., Int. Ed., 2008, 47, 6041–6044 CrossRef CAS PubMed.
- S. Huijser, E. H. Nejad, R. Sablong, C. de Jong, C. E. Koning and R. Duchateau, Macromolecules, 2011, 44, 1132–1139 CrossRef CAS.
- D. J. Darensbourg, R. R. Poland and C. Escobedo, Macromolecules, 2012, 45, 2242–2248 CrossRef CAS.
- T. Aida and S. Inoue, J. Am. Chem. Soc., 1985, 107, 1358–1364 CrossRef CAS.
- T. Aida, Y. Maekawa, S. Asano and S. Inoue, Macromolecules, 1988, 21, 1195–1202 CrossRef CAS.
- S. Takenouchi, A. Takasu, Y. Inai and T. Hirabayashi, Polym. J., 2002, 34, 36–42 CrossRef CAS.
- Z. J. Hua, G. R. Oi and S. Chen, J. Appl. Polym. Sci., 2004, 93, 1788–1792 CrossRef CAS.
- R. C. Jeske, A. M. DiCiccio and G. W. Coates, J. Am. Chem. Soc., 2007, 129, 11330–11331 CrossRef CAS PubMed.
- A. M. DiCiccio and G. W. Coates, J. Am. Chem. Soc., 2011, 133, 10724–10727 CrossRef CAS PubMed.
- E. H. Nejad, A. Paoniasari, C. E. Koning and R. Duchateau, Polym. Chem., 2012, 3, 1308–1313 RSC.
- E. H. Nejad, C. G. W. van Melis, T. J. Vermeer, C. E. Koning and R. Duchateau, Macromolecules, 2012, 45, 1770–1776 CrossRef CAS.
- E. H. Nejad, A. Paoniasari, C. G. W. van Melis, C. E. Koning and R. Duchateau, Macromolecules, 2013, 46, 631–637 CrossRef CAS.
- C. Robert, F. de Montigny and C. M. Thomas, Nat. Comm., 2011, 2, 586, DOI:10.1038/ncomms1596.
- C. Robert, T. Ohkawara and K. Nozaki, Chem.–Eur. J., 2014, 20, 4789–4795 CrossRef CAS PubMed.
- P. A. Vigato, V. Peruzzo and S. Tamburini, Coord. Chem. Rev., 2012, 256, 953–1114 CrossRef CAS PubMed.
- P. A. Vigato and S. Tamburini, Coord. Chem. Rev., 2008, 252, 1871–1995 CrossRef CAS PubMed.
- S. Huijser, B. B. P. Staal, J. Huang, R. Duchateau and C. E. Koning, Biomacromolecules, 2006, 7, 2465–2469 CrossRef CAS PubMed.
- S. Huijser, B. B. P. Staal, J. Huang, R. Duchateau and C. E. Koning, Angew. Chem., Int. Ed., 2006, 45, 4104–4108 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXL-97: Program for Crystal Structure Refinement, Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SADABS, University of Göttingen, 1996 Search PubMed.
- N. A. Law, T. E. Machonkin, J. P. McGorman, E. J. Larson, J. W. Kampf and V. L. Pecoraro, Chem. Commun., 1995, 2015–2016 RSC.
- M. R. Bermejo, A. Castineiras, J. C. Garcia-Monteagudo, M. Rey, A. Sousa, M. Watkinson, C. A. McAuliffe, R. G. Pritchard and R. L. Beddoes, Dalton Trans., 1996, 2935–2944 RSC.
- T. Aida and S. Inoue, Acc. Chem. Res., 1996, 29, 39–48 CrossRef CAS.
- W. J. van Meerendonk, R. Duchateau, C. E. Koning and G. J. M. Gruter, Macromolecules, 2005, 38, 7306–7313 CrossRef CAS.
- N. Nomura, A. Taira, A. Nakase, T. Tomioka and M. Okada, Tetrahedron, 2007, 63, 8478–8484 CrossRef CAS PubMed.
- J. Liu, Y. Y. Bao, Y. Liu, W. M. Ren and X. B. Lu, Polym. Chem., 2013, 4, 1439–1444 RSC.
- D. J. Darensbourg, R. M. Mackiewicz, J. L. Rodgers, C. C. Fang, D. R. Billodeaux and J. H. Reibenspies, Inorg. Chem., 2004, 43, 6024–6034 CrossRef CAS PubMed.
- A. Bernard, C. Chatterjee and M. H. Chisholm, Polymer, 2013, 54, 2639–2646 CrossRef CAS PubMed.
- D. F. Liu, X. M. Zhang, L. Q. Zhu, J. Wu and X. Q. Lü, Catal. Sci. Technol., 2015, 5, 562–571 RSC.
- N. J. Van Zee and G. W. Coates, Chem. Commun., 2014, 50, 6322–6325 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The syntheses and characterization of HL0, H2Ln and complexes 1–4 are founded in the ESI. The detailed copolymerization and the characterization of the copolymers were also founded in the ESI. The crystallographic data for [1(EtOH)] founded in Tables 1S and 2S. CCDC 924419. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra08969c |
| This journal is © The Royal Society of Chemistry 2015 |