Remarkable isomer-selective gelation of aromatic solvents by a polymorph of a urea-linked bile acid–amino acid conjugate†
Abstract
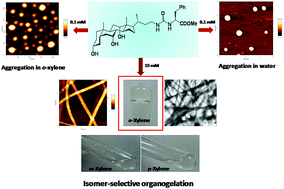
We report an unusual, isomer-selective gelation of aromatic solvents by a polymorph of a urea-linked bile acid–amino acid conjugate. The gelator showed selectivity towards gelation of 1,2-disubstituted aromatic solvents.

 Please wait while we load your content...
Please wait while we load your content...