Photocatalytic reduction of Cr(vi) by polyoxometalates/TiO2 electrospun nanofiber composites†
Abstract
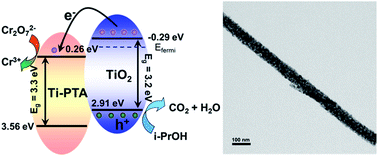
Polyoxometalates (Ti0.75PW12O40, Ti-PTA)/TiO2 nanofiber composites were fabricated by a simple electrospinning technique and then high temperature calcination. In the structure, Ti-PTA, as an electron relay, can accept the photo-generated electrons from the conduction band of TiO2, which promote the separation of photo-generated charges in TiO2. Then the electrons stored on Ti-PTA further transfer to the Cr(VI) in the solution to realize the removal of Cr(VI). The Ti-PTA/TiO2 nanofiber composites exhibit enhanced photocatalytic performance for photocatalytic reduction of Cr(VI), which might be a potential photocatalyst for Cr(VI) removal in environmental therapy.

 Please wait while we load your content...
Please wait while we load your content...