Systematic investigation of quinoxaline derivatization of sialic acids and their quantitation applicability using high performance liquid chromatography†
Abstract
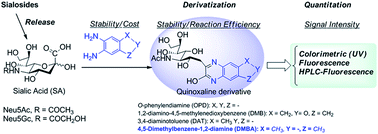
Quinoxaline derivatization has been the most commonly used approach for sialic acid quantitation of biological samples and glycoproteins by either HPLC or LC-MS/MS. However, the reported methods still suffer from many disadvantages, such as instable/expensive reagents, instable derivatives, and/or multiple products, which prevent their practical applications for SA-focused clinical screening and biopharmaceutical analysis. In this study, we investigated the quinoxaline derivatization of SAs with different commercially available phenyldiamines, and found a stable and inexpensive 4,5-dimethylbenzene-1,2-diamine (DMBA), that selectively reacted with SA to form a stable quinoxaline derivative efficiently and with strong UV and fluorescence absorption. The optimized DMBA derivatization conditions were successfully applied to quantitation of free and total SAs (N-acetyl-D-neuraminic acid (Neu5Ac) and N-glycolyl-D-neuraminic acid (Neu5Gc)) in fetal bovine serum (FBS) and glycoprotein fetuin using HPLC with fluorescence detection. The limit of detection (LOD) for Neu5Ac and Neu5Gc were 6.00 and 8.80 pg, and the limits of quantitation (LOQ) for Neu5Ac and Neu5Gc were 18.0 and 29.0 pg on column, respectively. This method was also applied to determine total SA in human plasma samples. This DMBA derivatization method provides a robust, easy-handling, sensitive and cost effective approach for quantitation of SAs in glycoconjugates and biomatrices.

 Please wait while we load your content...
Please wait while we load your content...