Hydrogen-bonding driven luminescent assembly and efficient Förster Resonance Energy Transfer (FRET) in a dialkoxynaphthalene-based organogel†
Abstract
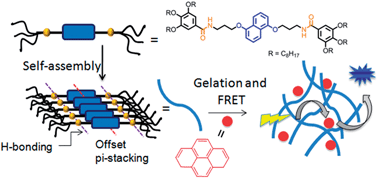
This paper reports self-assembly, photophysical studies and energy transfer in a bis-amide functionalized dialkoxynaphlane (DAN)-based organogel. Due to the synergistic effect of hydrogen bonding, π–π stacking and hydrophobic interaction, DAN showed long-range ordering in a non-polar solvent, methylcyclohexane (MCH), as evidenced by the fibrous morphology observed in TEM. It showed gelation in various organic solvents with very low critical gelation concentration (in some solvents as low as <0.1 wt%, suggesting very strong gelating propensity). UV/vis and photoluminescence studies of the gel indicated offset π-stacking between the DAN chromophores. Gel to sol transformation was noticed in presence of trace amount of H-bond competing solvent MeOH, indicating strong influence of H-bonds on gelation. Direct evidence for hydrogen bonding was further probed by a variable temperature NMR study in which the NH peak corresponding to the amide functionality was significantly upfield shifted at elevated temperature, signifying breakage of the assembly. Gelation induced enhanced emission was noticed which was attributed to offset π-stacking. As DAN and pyrene are known to form an efficient D–A pair for FRET, photoluminescence properties of the gel were tested in presence of pyrene. It was found that the fluorescence from the donor was completely quenched. In turn strong emission was observed from pyrene suggesting a very efficient FRET process possibly due to close proximity of the pyrene acceptor and the DAN chromophore in the gel state.
- This article is part of the themed collection: Supramolecular chemistry: self-assembly and molecular recognition

 Please wait while we load your content...
Please wait while we load your content...