Selective extraction of scandium from yttrium and lanthanides with amic acid-type extractant containing alkylamide and glycine moieties
Yuzo Babaa,
Arisa Fukamia,
Fukiko Kubotaa,
Noriho Kamiyaab and
Masahiro Goto*ab
aDepartment of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Fukuoka 819-0395, Japan. E-mail: m-goto@mail.cstm.kyushu-u.ac.jp; Fax: +81-92-802-2810; Tel: +81-92-802-2806
bCenter for Future Chemistry, Kyushu University, Fukuoka 819-0395, Japan
First published on 2nd October 2014
Abstract
The liquid–liquid extraction of rare earth metal ions (scandium (Sc3+), yttrium (Y3+) and the lanthanides (La3+, Nd3+, Eu3+ and Dy3+)) was investigated using N-[N,N-di(2-ethylhexyl)aminocarbonylmethyl]glycine (D2EHAG). Scandium was extracted selectively from lanthanides under highly acidic conditions (0 < pH ≤ 1.5), and stripped easily using a mild acidic solution such as 1 mol dm−3 H2SO4. By comparing the extraction behavior with N,N-dioctyldiglycol amic acid, which has a similar molecular structure to D2EHAG, or the commercial alkyl monocarboxylic acid extractant, Versatic 10, it was concluded that the affinity of D2EHAG to scandium was caused by a chelating effect and the size recognition ability of D2EHAG. The extraction mechanism was examined, and it was proven that the trivalent scandium ion is extracted by forming a stable metal complex with four D2EHAG molecules.
Introduction
Scandium is an important metal in high-technology industries because scandium and its compounds have been applied widely in the optical, electronic, aeronautical, automotive and transportation industries.1 Demand for this metal has therefore increased in recent years. Scandium is classified as a rare earth element together with yttrium and the lanthanides.2–5 Among the rare earth elements, scandium is expensive because it is scarce and exists in low concentrations in known deposits. The metallurgical processes for its purification and recovery are complex and effective scandium separation techniques are strongly desired.Liquid–liquid extraction (solvent extraction) is one of the most effective methods for the separation and purification of various metal ions.6,7 In this method, the extractant used plays a key role and the selection of an appropriate extractant often determines the success of the extraction process.7–10 A variety of extractants have been designed for the separation of critical metals including rare earth metals. Among them, industrially available organophosphorus extractants, such as di(2-ethyhexyl)-phosphoric acid (D2EHPA) and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A), have been used widely in commercial extraction processes.7 However, certain disadvantages still remain in the process; a high acid concentration is required for the stripping of certain target metals, poor selectivity occurs when certain combinations of metals need to be separated from one another, and problematic phosphorous residue may result as a secondary waste.11 Alkyl carboxylic acids such as Versatic 10 (neodecanoic acid) have also been used commercially; however, the extraction at a high pH range because of the high dissociation constant of carboxylic acid is disadvantageous in the effectiveness of the solvent extraction process.1
Many researchers have focused on developing novel extractants to solve these problems.11–16 A recently developed extractant is N,N-dioctyldiglycol amic acid (DODGAA).11,17 DODGAA has a tridentate structure framed with an amide group and a carboxyl group connected by an ether chain, which results in its high affinity for rare earth metals over other common metals. More recently, we have developed another novel extractant, N-[N,N-di(2-ethylhexyl)-aminocarbonylmethyl]glycine (D2EHAG),18 which is also a tridentate ligand framed with an amide group and a carboxyl group and connected by a secondary amino group (–NH–). Like DODGAA, this compound is also free of phosphorus. Previously, we used D2EHAG to separate Co and Ni metal ions from Mn. These have been difficult to separate using conventional extractants such as D2EHPA, because of their high selectivity to Mn over Co and Ni. Thus, D2EHAG, which can change the selectivity in a combination of metal ions, would be effective for some metal ions including scandium.
In this study, we examine the extraction of rare earth metals by D2EHAG, and focus on the extraction properties of Sc3+. The extraction behavior of Sc3+ with D2EHAG was compared to that with other extractants, including DODGAA.
Experimental
Chemicals
The D2EHAG extractant (Fig. 1(a)) was synthesized by a two step SN2 reaction as described previously.18 Briefly, 2-chloro-N,N-di(2-ethylhexyl)acetamide (CDEHAA) was synthesized by adding chloroacetyl chloride to a dichloromethane solution of di-(2-ethylhexyl)amine and triethylamine followed by stirring for 3 h at room temperature. The resultant solution was washed with 0.1 M HCl and deionized water, and dried with anhydrous sodium sulfate. Yellow liquid CDEHAA was obtained after filtration and solvent removal in vacuo. CDEHAA (12.7 g, 0.04 mol) was added dropwise to a methanol solution of glycine (15.0 g, 0.2 mol) and sodium hydroxide (8.0 g, 0.2 mol), and the reactant was stirred for 15 h at 333 K. After removing the solvent in vacuo, the residue was dissolved in dichloromethane and washed with 1 mol dm−3 H2SO4. The organic phase was washed with deionized water several times. Through the same purification step as that described for CDEHAA, a yellow viscous liquid was obtained (12.5 g, yield 87%). D2EHAG: 1H NMR (300 MHz, CDCl3) δ 8.83 (br, 1H), 4.04 (s, 2H), 3.74–2.80 (m, 6H), 1.60 (m, 2H), 1.25 (m, 16H), 0.88 (m, 12H). 13C NMR (75.5 MHz, CDCl3) δ 170.4, 165.9, 50.1, 48.2, 47.6, 37.7, 36.6, 30.5, 28.8, 23.8, 23.4, 14.1, 11.0, 10.9. Anal. calcd for C20H40N2O3·0.2H2O C, 66.70; H, 11.31; N, 7.78. Found: C, 66.71; H, 11.30; N, 7.49.For the comparison with D2EHAG, DODGAA (Fig. 1(b)) was synthesized as described previously.11,17 Versatic 10 (Fig. 1(c)) was supplied by Mitsubishi Chemical Co., (Tokyo, Japan).
Scandium(III) nitrate tetrahydrate was from Nacalai Tesque Inc. (Kyoto, Japan). Dysprosium(III) nitrate pentahydrate, and yttrium(III), lanthanum(III), europium(III) and neodymium(III) nitrate hexahydrates were from Kishida Chemical Co., Ltd. (Osaka, Japan). Special grade n-dodecane was used as an organic solvent.
The deionized water used in the experiments was purified with a Millipore Milli-Q system. All other reagents and solvents were of analytical grade and were used as received.
Extraction procedure
The extracting organic solution was prepared by dissolving the D2EHAG in n-dodecane, which is similar to industrial solvent kerosene. When DODGAA was used as an extractant, 1-octanol (5 vol%) was added to the n-dodecane phase as a solubilizer. An aqueous solution containing 0.1 mmol dm−3 rare earth metal ions was prepared by dissolving their nitrates in 0.1 mol dm−3 HNO3 or NH4NO3. The pH of the aqueous feed solution was adjusted by mixing these aqueous solutions and/or by adding 28 wt% ammonia solution.Equal volumes (5 cm3) of aqueous and organic solutions were mixed in a sealed tube using a vortex mixer and shaken gently for more than 1 hour to attain extraction equilibrium at 298 K. (We experimentally confirmed that rare earth metal ions were not soluble in n-dodecane at all without an extractant.)
After phase separation, the metal ions in the organic phase were stripped using an acidic solution. Stripping test was performed using equal volumes (3 cm3) of acidic aqueous and metal loaded organic solutions, and shaken intensely for 30 minutes after mixing solutions. The metal concentrations in the aqueous phase for the extraction and stripping tests were measured using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, Optima 5300, Perkin Elmer Co, Waltham, Ma, USA). The pH was measured using a pH meter (HM-30R, DKK-TOA Co, Tokyo, Japan).
The extent of extraction and stripping of the metal ions, E [−] and S [−], respectively, were calculated using eqn (1) and (2):
 | (1) |
 | (2) |
The distribution ratio D[−] was calculated from eqn (3):
 | (3) |
In a loading test, an organic phase containing 0.6 mmol dm−3 D2EHAG and aqueous phases with varying Sc3+ concentrations were equilibrated at a constant pH. In Job's continuous variation method, the total concentration of Sc3+ and D2EHAG was maintained at 100 mmol dm−3 and the extraction equilibrium was measured at various molar ratios of D2EHAG to the total concentration.
Results and discussion
Extraction behavior of metal ions
The extraction behavior of the rare earth metal ions (Sc3+, yttrium (Y3+) and the lanthanides (La3+, Nd3+, Eu3+ and Dy3+)), by D2EHAG in n-dodecane is shown in Fig. 2(a) as a function of equilibrium pH in the feed aqueous phase. Metal ion extraction was promoted with increasing pH, and quantitative extraction was achieved around pH 1 for Sc3+. The other rare earth metal ions were extracted at a pH from 1.5 to 3.0 to reach quantitative extraction. D2EHAG extracts Sc3+ selectively and permits its efficient separation from other rare earth metal ions. The extraction behavior of the metal ions was compared with that of DODGAA, which has a similar structure to D2EHAG except that a nitrogen atom in the center of D2EHAG is replaced by an oxygen atom.As shown in Fig. 2(b), Sc3+ was extracted together with other rare earth metal ions such as Y3+ and other lanthanide ions in almost the same pH range. Fig. 2(c) shows the extraction behavior of rare earth metal ions by a commercial extractant, Versatic 10, which is a typical alkyl monocarboxylic acid. Sc3+ was extracted at higher pH conditions, but its separation from other rare earth metal ions was possible. The higher extraction ability of D2EHAG or DODGAA for rare earth metal ions than that of Versatic 10 occurs because of the chelate effect created by the tridentate coordination structure with an amide and a carboxylic acid moiety. As described above, D2EHAG has a secondary amine at the center of the molecule instead of the ether oxygen of DODGAA, and this leads to its remarkably higher selectivity for Sc3+ compared with DODGAA. DODGAA appears to recognize a metal ion with a planar structure framed by the three oxygen atoms, while D2EHAG can capture the ion with a more flexible O, N, O frame in three dimensions. Therefore D2EHAG, which can recognize ion size more closely, is considered to be advantageous in that it can form a stable complex with Sc3+, for which the ionic radius is smaller than other rare earth metal ions.
Stripping of the rare earth metal ions from the extracting phase was also performed. Fig. 3 shows the stripping ratio, S, for the metal ions from the extracting phase prepared by extraction at pHeq = 3.5. As shown in Fig. 3, quantitative recovery could almost be attained using 1 mol dm−3 H2SO4, even for Sc3+, which was extracted under highly acidic conditions (0 < pH ≤ 1.5). Thus, it was demonstrated that D2EHAG can extract Sc3+ selectively from other rare earth metal ions.
Extraction mechanism
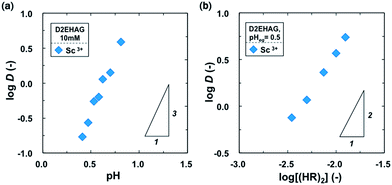
Slope analysis was conducted to investigate the extraction mechanism of Sc3+ with D2EHAG. Fig. 4(a) shows the relationship between the logarithmic distribution ratio of Sc3+, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D and pH. A linear correlation with a slope of 3 was obtained, indicating that three protons are released into the aqueous phase during the extraction of a trivalent scandium ion as described later.
D and pH. A linear correlation with a slope of 3 was obtained, indicating that three protons are released into the aqueous phase during the extraction of a trivalent scandium ion as described later.
 | ||
| Fig. 4 Extraction dependencies of Sc3+ on (a) pH with 10 mmol dm−3 D2EHAG, and (b) concentration of extractant dimer at pHeq = 0.5. | ||
The effect of extractant concentration on the extraction of Sc3+ was examined. Some alkyl monocarboxylic acids such as Versatic 10 exist as dimers in aliphatic solvents.19,20 Therefore D2EHAG is considered to be in dimer form, (HR)2,org, in n-dodecane. As shown in Fig. 4(b), plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D versus log(HR)2,org yield a linear correlation with a slope of 2, suggesting that one Sc3+ is extracted as a complex with four D2EHAG molecules into the organic phase.
D versus log(HR)2,org yield a linear correlation with a slope of 2, suggesting that one Sc3+ is extracted as a complex with four D2EHAG molecules into the organic phase.
A loading test was conducted to confirm the extraction stoichiometry. Fig. 5(a) shows plots of the molar ratio of initial D2EHAG concentration to loaded Sc3+ concentration in the organic phase as a function of the initial Sc3+ concentration. The plots decrease with increase in initial Sc3+ concentration to approach a constant value of 4. This result supports the idea that one trivalent scandium ion is extracted to the organic phase with four D2EHAG molecules, that is, two D2EHAG dimers. Job's continuous variation method for the extraction of Sc3+ was examined as shown in Fig. 5(b). The extracted Sc3+ density reached a maximum when the molar ratio of D2EHAG concentration to total concentration of Sc3+ and D2EHAG was approximately 0.78. Therefore, the ratio of Sc3+ to D2EHAG in the metal complex was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
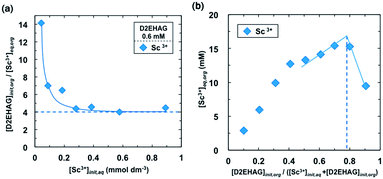 | ||
| Fig. 5 Binding-stoichiometry estimation of D2EHAG with Sc3+ (a) loading test (pHeq = 3.7), (b) Job's continuous variation method (pHeq = 3.0). | ||
Based on the analyses, the extraction of trivalent scandium by D2EHAG can be expressed by eqn (4):
| Scorg3+ + 2(HR)2,org ↔ (ScR3·(HR))org + 3H+ | (4) |
Conclusions
The extraction behavior of rare earth metals with a novel extractant, D2EHAG, was investigated. D2EHAG showed a significantly higher selectivity to Sc3+ than the other rare earth metal ions. DODGAA, which has a similar molecular structure to D2EHAG, exhibited little selectivity for Sc against heavy rare earth metal ions. The high affinity of D2EHAG for Sc3+, which has a smaller ionic radius than the other rare earth metal ions, was considered to be because of the size-recognition ability of D2EHAG with a flexible O, N, O tridentate extractant structure. Scandium was stripped easily using 1 mol dm−3 H2SO4. D2EHAG is therefore useful for the separation and recovery of scandium.Acknowledgements
This work was supported by The Environment Research and Technology Development Fund (ERTDF; grant number: 3K143014) from the Ministry of Environment, Japan. The authors thank Sumitomo Metal Mining Co., Ltd. for the financial support of this research. Y. Baba was supported by Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists (grant number: 12J05560).Notes and references
- W. Wang and C. Y. Cheng, J. Chem. Technol. Biotechnol., 2011, 86, 1237–1246 CrossRef CAS.
- Y. G. Wang, S. T. Yue, D. Q. Li, M. J. Jin and C. Z. Li, Solvent Extr. Ion Exch., 2002, 20, 701–716 CrossRef CAS PubMed.
- D. Wu, C. Niu, D. Li and Y. Bai, J. Alloys Compd., 2004, 374, 442–446 CrossRef CAS PubMed.
- M. Karve and B. Vaidya, Sep. Sci. Technol., 2008, 43, 1111–1123 CrossRef CAS.
- W. Wang, Y. Pranolo and C. Y. Cheng, Sep. Purif. Technol., 2013, 108, 96–102 CrossRef CAS PubMed.
- C. Xu, J. Wang and J. Chen, Solvent Extr. Ion Exch., 2012, 30, 623–650 CrossRef CAS.
- A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Chem. Soc. Rev., 2014, 43, 123–134 RSC.
- K. Binnemans, P. T. Jones, B. Blanpain, T. V. Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS PubMed.
- M. J. Hudson, L. M. Harwood, D. M. Laventine and F. W. Lewis, Inorg. Chem., 2013, 52, 3414–3428 CrossRef CAS PubMed.
- M. Iwakuma, T. Ohshima and Y. Baba, Solvent Extr. Res. Dev., Jpn., 2008, 15, 21–35 CAS.
- (a) H. Naganawa, K. Shimojo, H. Mitamura, Y. Sugo, J. Noro and M. Goto, Solvent Extr. Res. Dev., Jpn., 2007, 14, 151–159 CAS; (b) K. Shimojo, H. Naganawa, J. Noro, F. Kubota and M. Goto, Anal. Sci., 2007, 23, 1427–1430 CrossRef CAS.
- V. M. Jordanov, M. Atanassova and I. L. Dukov, Sep. Sci. Technol., 2002, 37, 3349–3356 CrossRef CAS PubMed.
- K. Ohto, M. Yano, K. Inoue, T. Yamamoto, M. Goto, F. Nakashio, S. Shinkai and T. Nagasaki, Anal. Sci., 1995, 11, 893–902 CrossRef CAS.
- Z. X. Zhu, Y. Sasaki, H. Suzuki, S. Suzuki and T. Kimura, Anal. Chim. Acta, 2004, 527, 163–168 CrossRef CAS PubMed.
- S. A. Ansari, P. Pathak, P. K. Mohapatra and V. K. Manchanda, Chem. Rev., 2012, 112, 1751–1772 CrossRef CAS PubMed.
- H. Narita and M. Tanaka, Solvent Extr. Res. Dev., Jpn., 2013, 20, 115–121 CAS.
- (a) K. Shimojo, A. Nakai, H. Okamura, T. Saito, A. Ohashi and H. Naganawa, Anal. Sci., 2014, 30, 513–517 CrossRef CAS; (b) K. Shimojo, N. Aoyagi, T. Saito, H. Okamura, F. Kubota, M. Goto and H. Naganawa, Anal. Sci., 2014, 30, 263–269 CrossRef CAS; (c) F. Yang, F. Kubota, Y. Baba, N. Kamiya and M. Goto, J. Hazard. Mater., 2013, 254–255, 79–88 CrossRef CAS PubMed; (d) F. Kubota, Y. Shimobori, Y. Baba, Y. Koyanagi, K. Shimojo, N. Kamiya and M. Goto, J. Chem. Eng. Jpn., 2011, 44, 307–312 CrossRef CAS; (e) Y. Baba, F. Kubota, N. Kamiya and M. Goto, Solvent Extr. Res. Dev., Jpn., 2011, 18, 193–198 CAS.
- Y. Baba, F. Kubota, N. Kamiya and M. Goto, Ind. Eng. Chem. Res., 2014, 53, 812–818 CrossRef CAS.
- J. S. Preston, Hydrometallurgy, 1985, 14, 171–188 CrossRef CAS.
- D. K. Singh, H. Singh and J. N. Mathur, Hydrometallurgy, 2006, 81, 174–181 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



