Chemodosimeter approach for nanomolar detection of Cu2+ ions and their bio-imaging in PC3 cell lines†
Shahi Imam Rejaa,
Vandana Bhallaa,
Shaffi Manchandab,
Gurcharan Kaurb and
Manoj Kumar*a
aDepartment of Chemistry, UGC Sponsored Centre for Advanced Studies-1, Guru Nanak Dev University, Amritsar, Punjab, India. E-mail: mksharmaa@yahoo.co.in; Fax: +91-183-2258820; Tel: +91-183-2258802 9 ext. 3205
bDepartment of Biotechnology, Guru Nanak Dev University, Amritsar, Punjab, India
First published on 1st September 2014
Abstract
A new rhodamine–azaindole based receptor 4 has been synthesized which selectively senses Cu2+ ions via a fluorescence resonance energy transfer (FRET) process in acetonitrile while in mixed aqueous media it undergoes Cu2+ promoted hydrolysis among the various metal ions tested (Pb2+, Ba2+, Fe3+, Cd2+, Ag+, Zn2+, Hg2+, Cu2+, Ni2+, Co2+, Fe2+ and Mg2+). The time resolved fluorescence studies also support the Cu2+ promoted hydrolysis of receptor 4 in mixed aqueous media. The detection limit for Cu2+ ions has been found to be 20 nM. The potential biological application of probe 4 is evaluated for in vitro detection of copper ions in prostate cancer (PC3) cell lines.
Introduction
Copper is third most abundant essential heavy metal ion (after iron and zinc) present in the human body which plays an important role in various physiological processes.1 Copper, being both useful and cytotoxic, is a vital trace element for the activities of enzymes because of its redox-active nature.2 However, exposure to higher doses can be harmful, as it can irritate the nose, mouth, and eyes, and cause headaches, dizziness, nausea, and diarrhoea.3 The cytotoxicity induced by copper is due to the mutations in ATP7B gene in Wilson's disease has been well recognized.4 Unusual uptake of certain levels of Cu2+ by animals is known to cause gastrointestinal disease, hypoglycemia, and infant liver damage.5 The toxicity of copper ion is linked with the generation of reactive oxygen species (ROS) via Haber–Weiss and Fenton reactions, which inhibit the activities of antioxidant enzymes, and hence results in aggregation.6 Keeping in view the role played by copper in day to day life the selective detection of copper in trace amounts is important not only for environmental applications, but also for toxicity purposes in living organism.7 Several methods like atomic absorption spectrometry,8 inductively coupled plasma mass spectroscopy (ICPMS),9 inductively coupled plasma atomic emission spectrometry (ICP-AES),10 and fluorescence spectroscopy etc. have been used for the detection of Cu2+ ions in trace amounts in various samples. Among these methods, fluorescence method is one of the best for detecting Cu2+ ions owing to its simplicity, high sensitivity, ready availability and non-destructive nature and as a result many fluorescent sensors for Cu2+ ion have been reported in the literature.11 Further copper ions have a catalytic nature and promote the hydrolysis (amides, esters),12 oxidations, dethioacetalizations, rearrangements, and oxidative cyclization.13 Thus there is a lot of scope to develop copper induced reaction based chemodosimeters for selective detection of Cu2+ ions.14Our research work involves design, synthesis and evaluation of fluorogenic receptors for selective sensing of soft metal ions, anions and evaluation of their switching behaviour.15 Recently, we reported from our laboratory a pentaquinone based compound 1 (Chart 1) having imine structure which undergoes Hg2+ promoted hydrolysis in mixed aqueous media.16 In continuation of this work we were now interested in studying the molecular recognition behaviour of a system having a donor and acceptor (with significant spectral overlap) linked together through an imine linkage in the presence of soft metal ions. For this purpose we designed and synthesized chemosensor 4 having rhodamine as acceptor and azaindole as donor joined together through an imine linkage. The imine moiety is introduced in the chemosensor 4 because of its good binding affinity toward the soft metal ions. Azaindole is introduced because it emits in the range 400–520 nm and rhodamine absorbs in the 470–580 nm range, so there is a significant overlap between azaindole and rhodamine moieties and hence considerable possibility of fluorescence resonance energy transfer in the presence of soft metal ions. On the other hand receptor 4 also contains an imine linkage and the possibility of metal ion induced hydrolysis cannot be ruled out in mixed aqueous media. Interestingly we observed fluorescence resonance energy transfer from azaindole to rhodamine in presence of Cu2+ ions in acetonitrile, while Cu2+ promoted hydrolysis of receptor 4 in mixed aqueous media. The biological application of probe 4 was evaluated for in vitro detection of copper ions in prostate cancer (PC3) cell lines.
Experimental
General information
All reagents were purchased from Aldrich and were used without further purification. Acetonitrile (AR grade) was used to perform analytical studies. UV-vis spectra were recorded on a SHIMADZU UV-2450 spectrophotometer, with a quartz cuvette (path length 1 cm). The cell holder was thermostatted at 25 °C. The fluorescence spectra were recorded with a SHIMADZU 5301 PC spectrofluorimeter. Mass spectra were recorded on a Bruker MicroTof QII mass spectrometer. 1H and 13C spectra were recorded on a JEOL-FT NMR-AL 300 MHz spectrophotometer using DMSO-d6 and CDCl3 as a solvent and tetramethylsilane as the internal standard. Data are reported as follows: chemical shift in ppm (d), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad singlet), coupling constants J (Hz), integration and interpretation.UV-vis and fluorescence titrations
UV-vis and fluorescence titrations were performed on 5.0 & 1.0 μM solutions of ligand in CH3CN and CH3CN–H2O (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 9
0.5, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 & 7
2 & 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) buffered with HEPES, pH = 7.0. Typically, aliquots of freshly prepared M(ClO4)n (M = Hg2+, Pb2+, Ba2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, Fe3+, Fe2+, K+, Mg2+, Na+ and Li+; n = 1, 2 or 3) standard solutions (10−1 M to 10−3 M) in CH3CN were added to record the UV-vis and fluorescence spectra.
3, v/v) buffered with HEPES, pH = 7.0. Typically, aliquots of freshly prepared M(ClO4)n (M = Hg2+, Pb2+, Ba2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, Fe3+, Fe2+, K+, Mg2+, Na+ and Li+; n = 1, 2 or 3) standard solutions (10−1 M to 10−3 M) in CH3CN were added to record the UV-vis and fluorescence spectra.
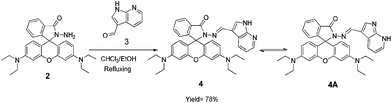
Synthesis of compound 3
A solution of rhodamine hydrazide 2 (100 mg, 0.219 mmol) and 7-azaindole-3-carboxaldehyde 3 (32 mg, 0.219 mmol) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dry dichloromethane and dry ethanol was refluxed for 24 h. After completion of the reaction, the solvent was evaporated and the residue left was crystallized from CHCl3–C2H5OH to give compound 4 in 78% yield; m.p. 213–215 °C; 1H NMR (DMSO-d6, 300 MHz) δ = 1.15 (t, J = 6 Hz, 12H, CH3), 3.32 (q, J = 6 Hz, 8H, CH2), 6.25 (d, J = 9 Hz, 2H, Ar-H), 6.51–6.45 (m, 4H, Ar-H), 7.04–7.0 (m, 1H, Ar-H), 7.19–7.17 (m, 1H, Ar-H), 7.43 (s, 1H, Ar-H), 7.54 (s, 2H, Ar-H), 7.94–7.91 (m, 1H, Ar-H), 9.36 (s, 1H, N
1 mixture of dry dichloromethane and dry ethanol was refluxed for 24 h. After completion of the reaction, the solvent was evaporated and the residue left was crystallized from CHCl3–C2H5OH to give compound 4 in 78% yield; m.p. 213–215 °C; 1H NMR (DMSO-d6, 300 MHz) δ = 1.15 (t, J = 6 Hz, 12H, CH3), 3.32 (q, J = 6 Hz, 8H, CH2), 6.25 (d, J = 9 Hz, 2H, Ar-H), 6.51–6.45 (m, 4H, Ar-H), 7.04–7.0 (m, 1H, Ar-H), 7.19–7.17 (m, 1H, Ar-H), 7.43 (s, 1H, Ar-H), 7.54 (s, 2H, Ar-H), 7.94–7.91 (m, 1H, Ar-H), 9.36 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 11.23 (s, 1H, NH) ppm. 13C NMR (DMSO-d6, 75 MHz): δ = 12.69, 43.81, 61.20, 97.62, 106.85, 108.30, 111.99, 114.23, 117.13, 122.93, 124.65, 128.34, 131.37, 133.88, 143.90, 148.65, 154.05, 188.34. ESI-MS (m/z) calcd for C36H36N6O2 calcd: 607.2792 (M + Na+) found: 607.2800 (M + Na+). Anal. calcd for C36H36N6O2: C 73.95, H 6.21, N 14.37; found C 74.12, H 6.42, N 14.29.
CH), 11.23 (s, 1H, NH) ppm. 13C NMR (DMSO-d6, 75 MHz): δ = 12.69, 43.81, 61.20, 97.62, 106.85, 108.30, 111.99, 114.23, 117.13, 122.93, 124.65, 128.34, 131.37, 133.88, 143.90, 148.65, 154.05, 188.34. ESI-MS (m/z) calcd for C36H36N6O2 calcd: 607.2792 (M + Na+) found: 607.2800 (M + Na+). Anal. calcd for C36H36N6O2: C 73.95, H 6.21, N 14.37; found C 74.12, H 6.42, N 14.29.
Results and discussion
Condensation of rhodamine hydrazide 2 with 7-azaindole-3-carboxaldehyde 3 in dry dichloromethane and absolute ethanol (10 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) gave the desired compound 4 in 78% yield (Scheme 1). The 1H NMR spectrum of compound 4 showed one triplet (12H) at 1.15 ppm which corresponds to methyl protons, one quartet (8H) at 3.32 ppm corresponding to methylene protons, one doublet (2H) at 6.25 ppm which corresponds to the aromatic protons, three multiplets (1H each) at 7.04–7.0, 7.19–7.17 and 7.94–7.91 ppm corresponding to the aromatic protons, one multiplet (4H) at 6.51–6.45 ppm which corresponds to the aromatic protons, four singlets (1H, 2H, 1H & 1H) at 7.43, 7.54, 9.36 and 11.23 ppm corresponding to the aromatic, imino and amine protons. The molecular ion peak at m/z 607.2800 [M + Na+] in the ESI-MS spectrum corresponds to the condensation product 4. These spectroscopic data corroborate the structure 4 for this compound (see pages S4–S6 ESI†).
1, v/v) gave the desired compound 4 in 78% yield (Scheme 1). The 1H NMR spectrum of compound 4 showed one triplet (12H) at 1.15 ppm which corresponds to methyl protons, one quartet (8H) at 3.32 ppm corresponding to methylene protons, one doublet (2H) at 6.25 ppm which corresponds to the aromatic protons, three multiplets (1H each) at 7.04–7.0, 7.19–7.17 and 7.94–7.91 ppm corresponding to the aromatic protons, one multiplet (4H) at 6.51–6.45 ppm which corresponds to the aromatic protons, four singlets (1H, 2H, 1H & 1H) at 7.43, 7.54, 9.36 and 11.23 ppm corresponding to the aromatic, imino and amine protons. The molecular ion peak at m/z 607.2800 [M + Na+] in the ESI-MS spectrum corresponds to the condensation product 4. These spectroscopic data corroborate the structure 4 for this compound (see pages S4–S6 ESI†).
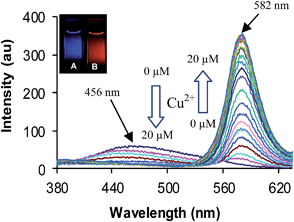
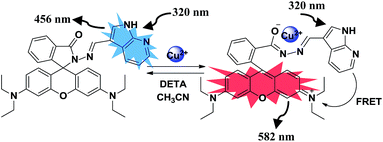
The binding behaviour of compound 4 was studied toward different metal ions by UV-vis and fluorescence spectroscopy. The absorption spectrum of compound 4 in acetonitrile (5.0 μM) shows two absorption bands at 278 and 320 nm. The band at 278 nm corresponds to S0–S1 transition while the band at 320 nm is due to the tautomer 4A (Fig. 1).17 Among the various metal ions tested (Pb2+, Ba2+, Fe3+, Cd2+, Ag+, Zn2+, Hg2+, Cu2+, Ni2+, Co2+, Fe2+ and Mg2+) only addition of Cu2+ ions leads to a change in UV-vis spectrum (see Fig. S7 ESI†). On addition of a 25 μM of Cu2+ ions to the solution of receptor 4 in CH3CN a new absorption band appears at 554 nm corresponding to the ring opened form of the rhodamine, while the bands at 278 and 320 nm show decrease in absorption. The characteristic absorption band of rhodamine is attributed to the opening of spirolactam ring to its amide form along with a colour change from colourless to pink of receptor 4. Upon addition of increasing amounts Cu2+ ions to the solutions of receptor 4 in acetonitrile a new emission band appeared at 582 nm along with gradual decrease of emission band at 456 nm (Fig. 2).18
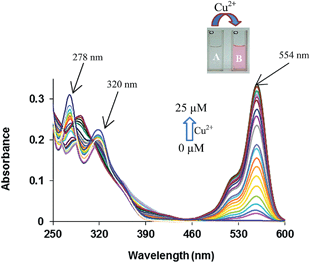 | ||
| Fig. 1 UV-vis spectra of 4 (5.0 μM) in the presence of Cu2+ ions (0–25 μM) in CH3CN; inset showing the colour change (A) before and (B) after the addition of Cu2+ ions. | ||
The intensity of the emission band at 582 nm increases with increase in concentration of Cu2+ ions. This is attributed to the binding of Cu2+ ions with spirolactam ring of rhodamine resulting in its opening to amide form. Thus, in the presence of Cu2+ ions efficient spectral overlap takes place between donor (azaindole moiety) and acceptor (rhodamine moiety) (Fig. 3) enhancing the intramolecular FRET (Scheme 2). This type of ratiometric fluorescence behaviour is not observed in the presence of any other metal ions (see Fig. S8 ESI†), except in the presence of Hg2+ and Fe3+ ions where a weak FRET behaviour is observed (see Fig. S9 ESI†). Fitting the changes in the fluorescence spectra of 4 with Cu2+ ions in acetonitrile, the nonlinear regression analysis program SPECFIT gave a good fit and demonstrated that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (host
1 stoichiometry (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) was the most stable species in the solution with a binding constant of (log
guest) was the most stable species in the solution with a binding constant of (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) = 4.60 with (±0.05 error). The method of continuous variation (Job's plot) was also used to prove the 1
β) = 4.60 with (±0.05 error). The method of continuous variation (Job's plot) was also used to prove the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry19 (see Fig. S10 ESI†). Thus, receptor 4 behaves as an efficient colorimetric and fluorogenic chemosensor for the detection of Cu2+ ions in acetonitrile (Fig. 1 & 2). However for biological applications, a chemosensor must work in aqueous/mixed aqueous media. Keeping this in view we studied the binding behaviour of chemosensor 4 in mixed aqueous media. The fluorescence spectrum of receptor 4 in CH3CN–H2O (7
1 stoichiometry19 (see Fig. S10 ESI†). Thus, receptor 4 behaves as an efficient colorimetric and fluorogenic chemosensor for the detection of Cu2+ ions in acetonitrile (Fig. 1 & 2). However for biological applications, a chemosensor must work in aqueous/mixed aqueous media. Keeping this in view we studied the binding behaviour of chemosensor 4 in mixed aqueous media. The fluorescence spectrum of receptor 4 in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) exhibits an emission band at 462 nm when excited at 320 nm. Upon addition of increasing amounts of Cu2+ ions (0–20 μM) to the solution of receptor 4 in CH3CN–H2O (7
3, v/v) exhibits an emission band at 462 nm when excited at 320 nm. Upon addition of increasing amounts of Cu2+ ions (0–20 μM) to the solution of receptor 4 in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) there was no change in the emission band at 462 nm and no new emission band was observed at 582 nm when excited at 320 nm. This indicates that there is no fluorescence resonance energy transfer from azaindole to rhodamine in mixed aqueous media (CH3CN–H2O, 7
3, v/v) there was no change in the emission band at 462 nm and no new emission band was observed at 582 nm when excited at 320 nm. This indicates that there is no fluorescence resonance energy transfer from azaindole to rhodamine in mixed aqueous media (CH3CN–H2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; v/v). However, when the fluorescence titration was repeated by exciting the solution of receptor 4 at 530 nm in mixed aqueous media (CH3CN–H2O, 7
3; v/v). However, when the fluorescence titration was repeated by exciting the solution of receptor 4 at 530 nm in mixed aqueous media (CH3CN–H2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
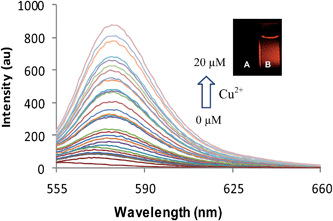
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) a new fluorescence emission band appeared at 582 nm upon addition of increase amount of Cu2+ ions (Fig. 4). The intensity of this emission band increased with increasing the concentration of Cu2+ ions. This emission band is attributed to the binding of Cu2+ ions to the spirolactam ring of the rhodamine moiety resulting in its opening to amide form. To understand the whole process, we studied the fluorescence behaviour of receptor 4 with Cu2+ ions in different CH3CN–H2O mixtures (9.5
3, v/v) a new fluorescence emission band appeared at 582 nm upon addition of increase amount of Cu2+ ions (Fig. 4). The intensity of this emission band increased with increasing the concentration of Cu2+ ions. This emission band is attributed to the binding of Cu2+ ions to the spirolactam ring of the rhodamine moiety resulting in its opening to amide form. To understand the whole process, we studied the fluorescence behaviour of receptor 4 with Cu2+ ions in different CH3CN–H2O mixtures (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 & 9
0.5 & 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 5). On addition of Cu2+ ions (0–20 μM during 40 minutes) to the solution of receptor 4 in CH3CN–H2O (9.5
1, v/v) (Fig. 5). On addition of Cu2+ ions (0–20 μM during 40 minutes) to the solution of receptor 4 in CH3CN–H2O (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) the intensity of the fluorescence emission band at 458 nm decreases while a new emission band appears at 582 nm when excited at 320 nm. This indicates that fluorescence resonance energy transfer takes place from azaindole to rhodamine in CH3CN–H2O (9.5
0.5) the intensity of the fluorescence emission band at 458 nm decreases while a new emission band appears at 582 nm when excited at 320 nm. This indicates that fluorescence resonance energy transfer takes place from azaindole to rhodamine in CH3CN–H2O (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v/v) solvent system. Interestingly after 40 minutes the intensity of the emission band at 582 nm decreases continuously and ultimately reaches zero with simultaneous increase in emission band at 456 nm when excited at 320 nm. This decrease in emission band at 582 nm may be due to two reasons (i) ring closing of spirolactam ring of the rhodamine moiety in receptor 4 or (ii) hydrolysis of receptor 4 to give azaindole 3 and rhodamine hydrazide 2 as a result of which no FRET takes place. To investigate this we recorded the fluorescence spectra of same solution and observed an emission band at 582 nm when excited at 530 nm, which clearly indicates that rhodamine is present in the solution as its ring opened amide form but may not be linked to azaindole moiety. From this observation we may conclude that there is a strong possibility of metal induced hydrolysis of this receptor 4 in CH3CN–H2O solvent system. Further, we also carried out fluorescence titration of receptor 4 with Cu2+ ions in CH3CN–H2O (9
0.5, v/v) solvent system. Interestingly after 40 minutes the intensity of the emission band at 582 nm decreases continuously and ultimately reaches zero with simultaneous increase in emission band at 456 nm when excited at 320 nm. This decrease in emission band at 582 nm may be due to two reasons (i) ring closing of spirolactam ring of the rhodamine moiety in receptor 4 or (ii) hydrolysis of receptor 4 to give azaindole 3 and rhodamine hydrazide 2 as a result of which no FRET takes place. To investigate this we recorded the fluorescence spectra of same solution and observed an emission band at 582 nm when excited at 530 nm, which clearly indicates that rhodamine is present in the solution as its ring opened amide form but may not be linked to azaindole moiety. From this observation we may conclude that there is a strong possibility of metal induced hydrolysis of this receptor 4 in CH3CN–H2O solvent system. Further, we also carried out fluorescence titration of receptor 4 with Cu2+ ions in CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and same phenomenon was observed. In this case however, the decrease in emission band at 582 nm along with gradual increase in emission band at 458 nm was observed after 30 minutes and in CH3CN–H2O (8
1, v/v) and same phenomenon was observed. In this case however, the decrease in emission band at 582 nm along with gradual increase in emission band at 458 nm was observed after 30 minutes and in CH3CN–H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) it was observed after only 6 minutes. These studies indicate that the rate of metal induced hydrolysis increases with increase in percentage of water. To gain deeper insight into the whole process we recorded the mass spectrum of receptor 4 in the presence of Cu2+ ions in CH3CN–H2O (7
2, v/v) it was observed after only 6 minutes. These studies indicate that the rate of metal induced hydrolysis increases with increase in percentage of water. To gain deeper insight into the whole process we recorded the mass spectrum of receptor 4 in the presence of Cu2+ ions in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) mixture. In the mass spectrum we did not observe a peak corresponding to 4–Cu2+ complex rather we observed a peak at 443.23 corresponding to rhodamine moiety which indicates hydrolysis of receptor 4 has taken place in the presence of Cu2+ ions in mixed aqueous media (CH3CN–H2O, 7
3, v/v) mixture. In the mass spectrum we did not observe a peak corresponding to 4–Cu2+ complex rather we observed a peak at 443.23 corresponding to rhodamine moiety which indicates hydrolysis of receptor 4 has taken place in the presence of Cu2+ ions in mixed aqueous media (CH3CN–H2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (see S11 ESI†). To prove this we attempted to prepare 4–Cu2+ complex by treating receptor 4 with copper perchlorate ions in CH3CN–H2O (7
3) (see S11 ESI†). To prove this we attempted to prepare 4–Cu2+ complex by treating receptor 4 with copper perchlorate ions in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
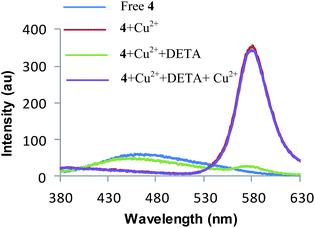
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The reaction was complete in 5 minutes (TLC). The solvent was then evaporated and residue so obtained was dissolved in CDCl3. The CDCl3 solution was passed through a Chelex resin to remove Cu2+ ions. The 1H NMR spectrum of the resulting solution shows the appearance of a singlet at 10.03 ppm corresponding to the proton of the aldehyde group of the azaindole (see page S14 ESI†). These spectroscopic studies clearly indicate that receptor 4 undergoes hydrolysis in the mixed aqueous media in the presence Cu2+ ions. Further, to confirm Cu2+ mediated hydrolysis, we carried out reversibility experiments in both CH3CN and CH3CN–H2O systems. The addition of diethylenetriamine (DETA) to the 4–Cu2+ complex in CH3CN restored the fluorescence signal of 4 to its original level (Fig. 6). Further addition of Cu2+ ions to the same solution gives fluorescence emission corresponding to the 4–Cu2+ complex which indicates the reversible behaviour of 4 in CH3CN. On the other hand, addition of DETA to the solution containing 4 and Cu2+ ions in CH3CN–H2O (9.5
3, v/v). The reaction was complete in 5 minutes (TLC). The solvent was then evaporated and residue so obtained was dissolved in CDCl3. The CDCl3 solution was passed through a Chelex resin to remove Cu2+ ions. The 1H NMR spectrum of the resulting solution shows the appearance of a singlet at 10.03 ppm corresponding to the proton of the aldehyde group of the azaindole (see page S14 ESI†). These spectroscopic studies clearly indicate that receptor 4 undergoes hydrolysis in the mixed aqueous media in the presence Cu2+ ions. Further, to confirm Cu2+ mediated hydrolysis, we carried out reversibility experiments in both CH3CN and CH3CN–H2O systems. The addition of diethylenetriamine (DETA) to the 4–Cu2+ complex in CH3CN restored the fluorescence signal of 4 to its original level (Fig. 6). Further addition of Cu2+ ions to the same solution gives fluorescence emission corresponding to the 4–Cu2+ complex which indicates the reversible behaviour of 4 in CH3CN. On the other hand, addition of DETA to the solution containing 4 and Cu2+ ions in CH3CN–H2O (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v/v) after 40 minutes did not alter the emission pattern of the 4–Cu2+ system when excited at 530 nm. The fluorescence emissions at 582 nm remained the same as was observed in the absence of DETA (see Fig. S13 ESI†). The complexation of DETA with Cu2+ ions did not affect the emission of highly fluorescent ring-opened rhodamine moiety generated during the Cu2+ ion promoted hydrolysis of receptor 4, which indicates that the reaction of 4 and Cu2+ ions in aqueous solution is irreversible in nature (Scheme 3). To study the effect of temperature on Cu2+ induced hydrolysis of receptor 4, we carried out temperature dependent fluorescence studies. It was found that the hydrolysis proceeds faster at high temperature (for example 50 °C) in comparison to hydrolysis at low temperature (25 °C). The rate constants (K25 and K50) at 25 and 50 °C were found to be 12.07 × 10−2 and 0.214015 s−1 respectively. The ratio of two rate constants (K50/K25) is 1.7731, which indicates that at high temperature reaction
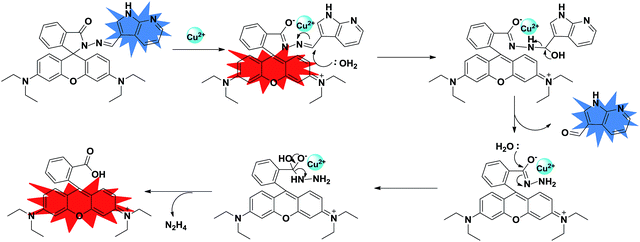
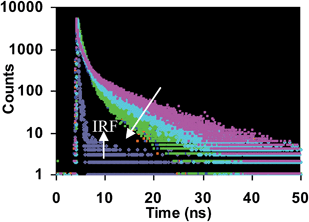
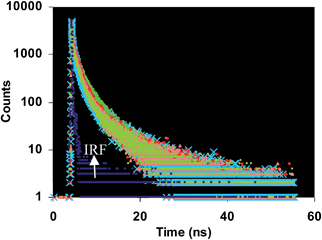
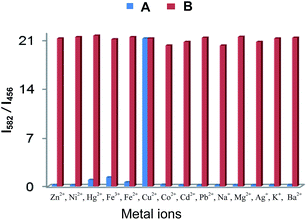
rate is fast compared to the reaction rate at low temperature (see S12 ESI†). Thus, from the above results we propose that the copper mediated hydrolysis of receptor 4 in mixed aqueous media proceeds in a stepwise manner. The first step of the mechanism involves binding of copper ion with the imino nitrogen atom of the receptor 4 as a result of which the electrophilicity of the imino carbon atom is enhanced which facilitates the attack by water molecule leading to formation of aldehyde and rhodamine hydrazide as products. In the next step the copper ion now binds with the rhodamine hydrazide derivative and the further attack of water molecule on the hydrazide derivative gives the final product as ring opened form of rhodamine B (Scheme 3). Further we also carried out the lifetime decay studies of the chemosensor 4 in acetonitrile and in mixed aqueous media. A picosecond time-resolved fluorescence technique was utilized to study the excited state behaviour of receptor 4 and 4–Cu2+ system in acetonitrile and mixed aqueous media. Single photon counting provides evidence for the non-radiative energy transfer from the donor to the acceptor moieties. Fluorescence lifetime decay profiles of donor moiety of receptor 4 was monitored (λex = 377 nm) in the presence of Cu2+ ions. The donor moiety shows fluorescence emission at 458 nm with highest single photon counting. The donor moiety showed shortening of decay time on increasing the concentration of Cu2+ ions (Fig. 7) in acetonitrile solvent and almost no change in decay time of the donor on addition of Cu2+ ions in mixed aqueous solvent (Fig. 8). The fluorescence lifetime decay of receptor 4 in (λex = 377 nm) exhibited triexponential decay (χ2 = 1.435) with lifetimes of 1.17 ns (35.45%), 5.85 ns (36.20%) and 0.03 ns (28.35%) in the absence of the Cu2+ ions in acetonitrile. In presence of 20 μM of the Cu2+ ions, 4 exhibited fast triexponential decay (χ2 = 1.562) with time constants of 0.87 ns (67.36%), 5.60 ns (17.42%) and 0.024 ns (17.42%). Such shortening in decay dynamics of the donor moiety in the presence of the acceptor clearly indicate the non-radiative energy transfer from azaindole to rhodamine moiety in acetonitrile (Fig. 7).20 On the other hand in mixed aqueous solvent the fluorescence lifetime decay of 4 in (λex = 377 nm) exhibited triexponential decay (χ2 = 1.684) with lifetimes of 1.13 ns (44.25%), 5.86 ns (36.15%) and 0.02 ns (19.60%) in the absence of the Cu2+ ions. In presence of 20 μM of the Cu2+ ions, 4 exhibited no such change in decay profile of the donor (Fig. 8) and exhibited triexponential decay (χ2 = 1.847) with time constants of 1.24 ns (43.29%), 5.50 ns (28.94%) and 0.009 ns (27.77%) as a result of which the energy transfer is ruled out and the metal induced hydrolysis was confirmed. To check the practical applicability of receptor 4 as a Cu2+ ions selective fluorescent sensor, we also carried out competitive experiments in the presence of Cu2+ at 20 μM mixed with Fe2+, Fe3+, Cu2+, Ni2+, Cd2+, Co2+, Pb2+, Zn2+, Ba2+, Ag+, K+, Na+ and Li+ at 20 μM and as shown in Fig. 9B, no significant variation was found in the fluorescence intensity by comparison with or without the other metal ions. It was found that receptor 4 has a detection limit21 of 20 nanomolar for Cu2+ ions (see S16 ESI†) in CH3CN–H2O (7
0.5, v/v) after 40 minutes did not alter the emission pattern of the 4–Cu2+ system when excited at 530 nm. The fluorescence emissions at 582 nm remained the same as was observed in the absence of DETA (see Fig. S13 ESI†). The complexation of DETA with Cu2+ ions did not affect the emission of highly fluorescent ring-opened rhodamine moiety generated during the Cu2+ ion promoted hydrolysis of receptor 4, which indicates that the reaction of 4 and Cu2+ ions in aqueous solution is irreversible in nature (Scheme 3). To study the effect of temperature on Cu2+ induced hydrolysis of receptor 4, we carried out temperature dependent fluorescence studies. It was found that the hydrolysis proceeds faster at high temperature (for example 50 °C) in comparison to hydrolysis at low temperature (25 °C). The rate constants (K25 and K50) at 25 and 50 °C were found to be 12.07 × 10−2 and 0.214015 s−1 respectively. The ratio of two rate constants (K50/K25) is 1.7731, which indicates that at high temperature reaction
rate is fast compared to the reaction rate at low temperature (see S12 ESI†). Thus, from the above results we propose that the copper mediated hydrolysis of receptor 4 in mixed aqueous media proceeds in a stepwise manner. The first step of the mechanism involves binding of copper ion with the imino nitrogen atom of the receptor 4 as a result of which the electrophilicity of the imino carbon atom is enhanced which facilitates the attack by water molecule leading to formation of aldehyde and rhodamine hydrazide as products. In the next step the copper ion now binds with the rhodamine hydrazide derivative and the further attack of water molecule on the hydrazide derivative gives the final product as ring opened form of rhodamine B (Scheme 3). Further we also carried out the lifetime decay studies of the chemosensor 4 in acetonitrile and in mixed aqueous media. A picosecond time-resolved fluorescence technique was utilized to study the excited state behaviour of receptor 4 and 4–Cu2+ system in acetonitrile and mixed aqueous media. Single photon counting provides evidence for the non-radiative energy transfer from the donor to the acceptor moieties. Fluorescence lifetime decay profiles of donor moiety of receptor 4 was monitored (λex = 377 nm) in the presence of Cu2+ ions. The donor moiety shows fluorescence emission at 458 nm with highest single photon counting. The donor moiety showed shortening of decay time on increasing the concentration of Cu2+ ions (Fig. 7) in acetonitrile solvent and almost no change in decay time of the donor on addition of Cu2+ ions in mixed aqueous solvent (Fig. 8). The fluorescence lifetime decay of receptor 4 in (λex = 377 nm) exhibited triexponential decay (χ2 = 1.435) with lifetimes of 1.17 ns (35.45%), 5.85 ns (36.20%) and 0.03 ns (28.35%) in the absence of the Cu2+ ions in acetonitrile. In presence of 20 μM of the Cu2+ ions, 4 exhibited fast triexponential decay (χ2 = 1.562) with time constants of 0.87 ns (67.36%), 5.60 ns (17.42%) and 0.024 ns (17.42%). Such shortening in decay dynamics of the donor moiety in the presence of the acceptor clearly indicate the non-radiative energy transfer from azaindole to rhodamine moiety in acetonitrile (Fig. 7).20 On the other hand in mixed aqueous solvent the fluorescence lifetime decay of 4 in (λex = 377 nm) exhibited triexponential decay (χ2 = 1.684) with lifetimes of 1.13 ns (44.25%), 5.86 ns (36.15%) and 0.02 ns (19.60%) in the absence of the Cu2+ ions. In presence of 20 μM of the Cu2+ ions, 4 exhibited no such change in decay profile of the donor (Fig. 8) and exhibited triexponential decay (χ2 = 1.847) with time constants of 1.24 ns (43.29%), 5.50 ns (28.94%) and 0.009 ns (27.77%) as a result of which the energy transfer is ruled out and the metal induced hydrolysis was confirmed. To check the practical applicability of receptor 4 as a Cu2+ ions selective fluorescent sensor, we also carried out competitive experiments in the presence of Cu2+ at 20 μM mixed with Fe2+, Fe3+, Cu2+, Ni2+, Cd2+, Co2+, Pb2+, Zn2+, Ba2+, Ag+, K+, Na+ and Li+ at 20 μM and as shown in Fig. 9B, no significant variation was found in the fluorescence intensity by comparison with or without the other metal ions. It was found that receptor 4 has a detection limit21 of 20 nanomolar for Cu2+ ions (see S16 ESI†) in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), which is sufficiently low for the detection of nanomolar concentration range of Cu2+ ions found in many chemical systems. The fluorescence quantum yield (see S3 ESI†) of the 4 + Cu2+ system is 0.26 (at λem = 582 nm, λex = 530 nm) as compared to that of free 4 (0.02).
3, v/v), which is sufficiently low for the detection of nanomolar concentration range of Cu2+ ions found in many chemical systems. The fluorescence quantum yield (see S3 ESI†) of the 4 + Cu2+ system is 0.26 (at λem = 582 nm, λex = 530 nm) as compared to that of free 4 (0.02).
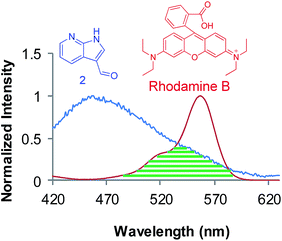 | ||
| Fig. 3 Spectral overlap between donor (3) emission (blue) and ring opened rhodamine B absorption (red). | ||
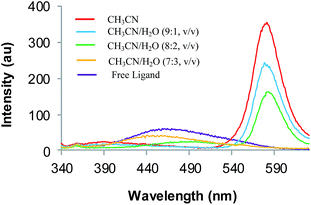 | ||
Fig. 5 Fluorescence spectra of 4 (1.0 μM) in response to the presence of Cu2+ ions (0–20 μM) in CH3CN, CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7 2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v); λex = 320 nm. 3 v/v); λex = 320 nm. | ||
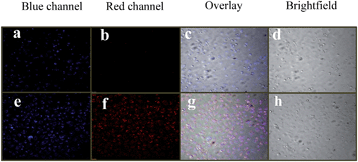
The potential biological application of 4 was evaluated for in vitro detection of Cu2+ ions in prostate cancer (PC3) cell lines. The prostate cancer (PC3) cell lines were incubated with probe 4 (1.0 μM) in an RPMI-1640 medium for 20 min at 37 °C and washed with phosphate buffered saline (PBS) buffer (pH 7.4) to remove excess of receptor 4. Microscope image did not exhibit any intracellular fluorescence in red channel which indicates the existence of ring closed rhodamine moiety. The cells with receptor 4 (1.0 μM) were then treated with Cu2+ ions (5.0 μM) in the RPMI-1640 medium and incubated again for 20 min at 37 °C and washed with PBS buffer. After treatment with Cu2+ ions, the cells show fluorescence in blue and red channels (Fig. 10e and f) which suggest that the probe 4 is cell permeable and an effective copper ion imaging agent with the appearance of blue and red coloured fluorescence emission attributed to the Cu2+ mediated hydrolysis of the probe 4, the red fluorescence emission corresponding to ring opened rhodamine moiety and the blue channel fluorescence emission due to the 7-azaindole-3-carboxyaldehyde product.
Conclusion
In summary, we synthesized a new rhodamine based fluorescence probe for Cu2+ ions which exhibits fluorescence resonance energy transfer in acetonitrile via Cu2+ induced spirolactam ring opening of rhodamine, whereas it undergoes Cu2+ induced hydrolysis in presence of water with time. Confocal microscopy images indicate that our probe can detect the level changes of Cu2+ ions in living cells.Acknowledgements
We are thankful to CSIR, UGC, and DST (New Delhi) for financial support. S. I. R. is thankful to UGC for senior research fellowship and Guru Nanak Dev University for providing research facilities.Notes and references
- (a) D. Y. Sasaki, D. R. Shnek, D. W. Pack and F. H. Arnold, Angew. Chem., Int. Ed. Engl., 1995, 34, 905–907 CrossRef CAS PubMed; (b) R. Krämer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef; (c) P. Grandini, F. Mancin, P. Tecilla, P. Scrimin and U. Tonellato, Angew. Chem., Int. Ed., 1999, 38, 3061–3064 CrossRef CAS; (d) L. Huang, X. Wang, G. Xie, P. Xi, Z. Li, M. Xu, Y. Wu, D. Bai and Z. Zeng, Dalton Trans., 2010, 39, 7894–7896 RSC.
- K. C. Ko, J. S. Wu, H. J. Kim, P. S. Kwon, J. W. Kim, R. A. Bartsch, J. Y. Lee and J. S. Kim, Chem. Commun., 2011, 47, 3165–3167 RSC.
- (a) T. R. Halfdanarson, N. Kumar, C. Y. Li, R. L. Phyliky and W. J. Hogan, Eur. J. Haematol., 2008, 80, 523–531 CrossRef CAS PubMed; (b) S. R. Jaiser and G. P. Winston, J. Neurol., 2010, 257, 869–871 CrossRef CAS PubMed.
- I. Sternlieb and I. H. Scheinberg, N. Engl. J. Med., 1968, 278, 352 CrossRef CAS PubMed.
- B. Sarkar, in Metal Ions in Biological System, ed. H. Siegel and A. Siegel, Marcel Dekker, New York, 1981, vol. 12, p. 233 Search PubMed.
- (a) D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed; (b) J. F. Zhang, Y. Zhou, J. Yoon, Y. Kim, S. J. Kim and J. S. Kim, Org. Lett., 2010, 12, 3852–3855 CrossRef CAS PubMed.
- J. Li, Y. Zeng, Q. Hu, X. Yu, J. Guo and Z. Pan, Dalton Trans., 2012, 41, 3623–3626 RSC.
- (a) A. P. S. Gonzales, M. A. Firmino, C. S. Nomura, F. R. P. Rocha, P. V. Oliveira and I. Gaubeur, Anal. Chim. Acta, 2009, 636, 198–204 CrossRef CAS PubMed; (b) N. Pourreza and R. Hoveizavi, Anal. Chim. Acta, 2005, 549, 124–128 CrossRef CAS PubMed.
- J. S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska and M. V. Zoriy, Anal. Chem., 2007, 79, 3208–3216 Search PubMed.
- Y. Liu, P. Liang and L. Guo, Talanta, 2005, 68, 25–30 CrossRef CAS PubMed.
- (a) T. Mistri, R. Alam, M. Dolai, S. K. Mandal, A. R. K. Bukhsh and M. Ali, Org. Biomol. Chem., 2013, 11, 1563–1569 RSC; (b) C. Kar, M. D. Adhikari, A. Ramesh and G. Das, Inorg. Chem., 2013, 52, 743–752 CrossRef CAS PubMed; (c) S. Adhikari, A. Ghosh, S. Mandal, A. Sengupta, A. Chattopadhyay, J. S. Matalobos, S. Lohar and D. Das, Dalton Trans., 2014, 43, 7747–7751 RSC; (d) J. Huang, M. Liu, X. Ma, Q. Dong, B. Ye, W. Wang and W. Zeng, RSC Adv., 2014, 4, 22964 RSC; (e) J. Huang, M. Tang, M. Liu, M. Zhou, Z. Liu, Y. Cao, M. Zhu, S. Liu and W. Zeng, Dyes Pigm., 2014, 107, 1–8 CrossRef CAS PubMed; (f) H. Zhao, Y. Wang, Z. Liu and B. Dai, RSC Adv., 2014, 4, 13161 RSC.
- (a) N. Li, Y. Xiang and A. Tong, Chem. Commun., 2010, 46, 3363–3365 RSC; (b) A. Mokhir and R. Krämer, Chem. Commun., 2005, 2244 RSC; (c) J. Kovács, T. Rödler and A. Mokhir, Angew. Chem., Int. Ed., 2006, 45, 7815 CrossRef PubMed; (d) J. Kovács and A. Mokhir, Inorg. Chem., 2008, 47, 1880 CrossRef PubMed.
- (a) V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS; (b) L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 10–11 CrossRef CAS PubMed; (c) X. Qi, E. J. Jun, L. Xu, S. J. Kim, J. Hong, Y. J. Yoon and J. Yoon, J. Org. Chem., 2006, 71, 2881–2884 CrossRef CAS PubMed; (d) M. H. Kim, H. H. Jang, S. Yi, S. K. Chang and M. S. Han, Chem. Commun., 2009, 4838–4840 RSC; (e) Q. Wu and E. V. Anslyn, J. Am. Chem. Soc., 2004, 126, 14682–14683 CrossRef CAS PubMed; (f) A. Basu and G. Das, Dalton Trans., 2011, 40, 2837–2843 RSC; (g) M. Yu, M. Shi, Z. Chen, F. Li, X. Li, Y. Gao, J. Xu, H. Yang, Z. Zhou, T. Yi and C. Huang, Chem.–Eur. J., 2008, 14, 6892–6900 CrossRef CAS PubMed; (h) W. Lin, L. Yuan, W. Tan, J. Feng and L. Long, Chem.–Eur. J., 2009, 15, 1030–1035 CrossRef CAS PubMed; (i) W. Lin, L. Long, B. Chen, W. Tan and W. Gao, Chem. Commun., 2010, 46, 1311–1313 RSC; (j) Y. Zhu and Y. Wei, Eur. J. Org. Chem., 2013, 4503–4508 CrossRef CAS PubMed.
- (a) H. S. Jung, J. H. Han, Y. Habata, C. Kang and J. S. Kim, Chem. Commun., 2011, 47, 5142–5144 RSC; (b) M. Yang, W. Meng, X. Liu, N. Su, J. Zhou and B. Yang, RSC Adv., 2014, 4, 22288 RSC; (c) C. Zhao, P. Feng, J. Cao, X. Wang, Y. Yang, Y. Zhang, J. Zhang and Y. Zhang, Org. Biomol. Chem., 2012, 10, 3104–3109 RSC.
- (a) M. Kumar, S. I. Reja and V. Bhalla, Org. Lett., 2012, 14, 6084–6087 CrossRef CAS PubMed; (b) S. I. Reja, N. Kumar, R. Sachdeva, V. Bhalla and M. Kumar, RSC Adv., 2013, 3, 17770 RSC; (c) M. Kumar, A. Dhir and V. Bhalla, Chem. Commun., 2010, 46, 6744–6746 RSC; (d) M. Kumar, A. Dhir and V. Bhalla, Eur. J. Org. Chem., 2009, 4534–4540 CrossRef CAS PubMed; (e) V. Bhalla, V. Vij, A. Dhir and M. Kumar, Chem.–Eur. J., 2012, 18, 3765–3772 CrossRef CAS PubMed; (f) M. Kumar, N. Kumar, V. Bhalla, P. R. Sharma and T. Kaur, Org. Lett., 2012, 14, 406–409 CrossRef CAS PubMed.
- V. Bhalla, Roopa, M. Kumar, P. R. Sharma and T. Kaur, Dalton Trans., 2013, 42, 15063–15068 RSC.
- (a) P. T. Chou, J. H. Liao, C. Y. Wei, C. Y. Yang, W. S. Yu and Y. H. Chou, J. Am. Chem. Soc., 2000, 122, 986–987 CrossRef CAS; (b) P. T. Chou, W. S. Yu, Y. C. Chen, C. Y. Wei and S. S. Martinez, J. Am. Chem. Soc., 1998, 120, 12927–12934 CrossRef CAS.
- P. W. Wu, W. T. Hsieh, Y. M. Cheng, C. Y. Wei and P. T. Chou, J. Am. Chem. Soc., 2006, 128, 14426–14427 CrossRef CAS PubMed.
- P. Job, Ann. Chim., 1928, 9, 113–203 CAS.
- C. Vijayakumar, V. K. Praveen and A. Ajayaghosh, Adv. Mater., 2009, 21, 2059–2063 CrossRef CAS PubMed.
- S. Goswami, S. Das, K. Aich, D. Sarkar, T. K. Mondal, C. K. Quah and H.-K. Fun, Dalton Trans., 2013, 42, 15113–15119 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR, mass, UV-vis and fluorescence spectra. See DOI: 10.1039/c4ra08894h |
| This journal is © The Royal Society of Chemistry 2014 |