DOI:
10.1039/C4RA08875A
(Paper)
RSC Adv., 2014,
4, 56690-56700
Simultaneous determination of heavy metals in biological samples by a multiple-template imprinting technique: an electrochemical study†
Received
19th August 2014
, Accepted 22nd October 2014
First published on 22nd October 2014
Abstract
In order to make people aware of the contamination of heavy metals in our daily life by inappropriate food intake, we have studied the contents of copper, cadmium, and lead in various components of our biota (soil, water, and plants, i.e. fruits and vegetables), collected from different areas (normal, industrial, coal-mine, river, and hill areas). For the accurate and rapid sensing of these metal ions, a multi-template imprinted nanowire modified electrochemical sensor was reported. The imprinted nanowire was synthesized using multiwalled carbon nanotubes as a core, on which a layer of conducting polyarginine is cast using an electro-polymerization technique. To study the total uptake of food-borne Cu(II), Cd(II), and Pb(II) ions in the human body, blood samples of six people were taken based on their different diets in their daily routine. Moreover, during the soil, water and plant analysis, it was observed that plants growing in a contaminated environment have a large accumulation of metal ions in their leaves and fruits. One more study was performed to explore ‘how much heavy metal we are taking in from our regular diet?’ and it was perceived that some of the food, which has lower concentrations of copper ions, can release a bigger amount of metal ions after digestion.
Introduction
Metals are a very vital and crucial part of our environment and/or ecosystem. However, following anthropogenic inputs or civilization, their levels become unbalanced, which can cause toxicity to our biota.1 The lethal effects of single metals on the environment have been fairly well documented and to assay them is also a simple errand. But the major problem of environmental pollution is associated with the collective effect of heavy metal ions, such as mercury, lead, cadmium, copper, etc. The U.S. Environmental Protection Agency (EPA) has recently recognized this issue of the heavy metal impact on ecosystem toxicity to be a large knowledge gap.2 The interpretation of the total impact of metal mixtures on soil and plants is complex because various types of chemical or physiological interactions are possible at the site of toxicity. These interactions depend on the type of metal, concentrations, time of stay in the system and environmental conditions. It is a very known fact that heavy metals can easily enter into the food chain and get accumulated in living organisms, causing various diseases and disorders.1 Different environmental protection agencies have defined the normal level of heavy metals, which is essential for all plants, animals and human beings. However, any elevation in this level can lead to toxic effects in plants and soil-dwelling animals and hence on ecosystems as a whole.3 For this reason, an ecological risk assessment of heavy metals and their combined effect on other metals is an important aspect of the management of concentrations of the metals in soils.
Several works have been reported so far for the estimation of metal ions by various techniques, such as liquid–liquid extraction,4 solid phase extraction,5 optical emission spectroscopy,6,7 stripping voltammetry,8 adsorptive voltammetry,9 and cloud point extraction10 in various samples and medium (water, soil, food, beverages, etc.). Among these, electrochemical techniques are most popular for the individual [e.g. Cu(II),11 Cd(II),12 Pb(II),13 Hg(II),14 As(III),15 Zn(II),16 and Se(IV)17] or simultaneous18–22 determination of heavy metal ions in real matrices. But the role of metal ions and their impact on these samples are not well explored yet.
In this work, our main focus is to study the variation in concentration of some heavy metal ions (copper, cadmium and lead) in various parts of our ecosystem, i.e. water, soil, plants, fruits and/or vegetables, in different areas (industrial, populated, hills, and river side). However, we are also trying to explore the release rate of metal ions in our body, after digestion. In this work, for sensitive, selective, and accurate determination of the concentration of these three metal ions, copper, cadmium, and lead, we have employed multi-template imprinted nanowires on the surface of multi-walled carbon nanotubes (MWCNTs) by electropolymerization technique. The synthesis of molecularly imprinted polymers (MIPs) for a single template is very common nowadays, but their application in a multi-template analysis is still under development.23,24 In case of multi-template imprinting, two or more template molecules get imprinted on a single polymer matrix. But there are several disadvantages in a multi-imprinting process, viz. template leakage, poor binding capability, etc., resulting in a lower popularity of this technique. To resolve this issue, herein, imprinted nanowires were synthesized having multi-walled carbon nanotubes (MWCNTs) as a core and polyarginine as a coating layer. The unique chemical, physical, and electronic properties of MWCNTs make them very suitable for the construction of electrochemical sensors as well as they improve the imprinting properties. Based on the popularity of MWCNTs, few works are already reported for the detection and separation of metal ions.25 But a multi-template analysis or simultaneous analysis of metal ions by imprinted nanowire modified MWCNTs is not yet reported.
In the present work, the synthesis of imprinted nanowire modified MWCNTs is successfully contemplated, where a layer of conducting polyarginine is cast on the MWCNT core using an electropolymerization technique. The MWCNTs were first modified with carboxylic groups to make the MWCNT surface polar. As a result, each nanotube (or small bundle) was wrapped with –COOH groups (hydrophilic groups) and coated onto the surface of the pencil graphite electrode (PGE). This was followed by the insertion and growth of arginine monomers along with the template molecules (metal ions; copper, cadmium, and lead) on the MWCNT surface via electropolymerization. The removal of the template molecules from the MWCNT–polyarginine matrix produces coaxial nanowire structures with a MWCNT center and conducting polyarginine as the coating. Cyclic voltammetry (CV) and differential pulse stripping voltammetry (DPSV) techniques were deployed for the simultaneous estimation of cadmium, copper, and lead using modified PGE.
Experimental
Reagents and apparatus
Cadmium sulfate, copper sulfate, lead oxide, pepsin from porcine gastric mucosa and pancreatin from porcine pancreas, MWCNTs (internal diameter of 2–6 nm, outer diameter of 10–15 nm, length of 0.2–10 μm, and purity >98.9%) and other interfering compounds were purchased from Sigma Aldrich Chemie (Steinheim, Germany). Sodium hydroxide (NaOH), sodium dihydrogen orthophosphate, disodium hydrogen phosphate, tetrahydrofuran (THF), sulfuric acid (H2SO4), nitric acid, hydrochloric acid, hydrogen peroxide, sodium bicarbonate, and ethanol were procured from Spectrochem Pvt. Ltd. (Mumbai, India). Pencil rods (0.5 mm × 5 cm) were purchased from Hi Par, Camlin, Mumbai. The stock solutions of Cd(II), Cu(II) and Pb(II) of 250 mg L−1 were prepared by dissolving the appropriate amounts of cadmium sulfate, copper sulfate and lead oxide in double distilled water. All of the electrochemical analysis was performed on a CH instrument (USA, model number 660C), using a three electrode cell assembly consisting of an imprinted nanowire-modified pencil graphite electrode (PGE), a platinum wire and Ag/AgCl (3.0 M KCl) as working, counter, and reference electrodes, respectively. The UV spectral analysis of the polymer was done using a Perkin Elmer Lambda-35 spectrophotometer. Morphological images of bare and modified electrode surfaces were recorded using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX), Hitachi, model S-3400 N. Heavy metal ions were also estimated by an atomic absorption spectrophotometer (AAS-GBC Avanta & GBC-902 including Graphite Furnace GBC GF 3000, Australia) as standard technique. All the experiments were performed at room temperature (25 ± 1 °C).
Computational study
All calculations have been carried out using the Gaussian 03 and Gauss View 4.0 software packages. Molecular structures were created with the chemdraw software and possible conformations of each molecule were optimized using the AM1 semi-empirical method. Then, the most stable conformations were optimized using the HF and ONIOM methods. To find out the most stable complex of the monomer (arginine) and metal ions, their different molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n; template
n; template![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer, where n = 1, 2, and 3) were designed and interaction energies were calculated using the following equation, where n refers to the monomer number in the template–monomer complexes:
monomer, where n = 1, 2, and 3) were designed and interaction energies were calculated using the following equation, where n refers to the monomer number in the template–monomer complexes:| | |
ΔE = E(template − monomer complex) − [E(template) + nE(monomer)]
| (1) |
Preparation of imprinted and non-imprinted nanowire modified PGEs
Prior to the electrode fabrication, pencil lead was pre-treated in 6.0 M nitric acid for 15 min, which was followed by water washing. After that, the lead was rubbed with cotton to clean the surface and dried at room temperature. The pencil lead (length 5 mm, diameter 0.5 mm) was housed in a micropipette tip, where electrical contact was obtained by wrapping a metallic wire on the one side of the lead and the other end was modified with MWCNTs. First, the –COOH group modified MWCNTs are drop-coated onto the PGE surface, followed by cyclic voltammetric runs (10 cycle) in the range of −2.0 to +2.5 V at a scan rate of 100 mV s−1 in an aqueous solution of phosphate buffer (1 mL, 0.03 M), arginine (6 mL, 0.1 M) and templates (1 mL of each salt, cadmium sulfate, copper sulfate and lead oxide) (Scheme 1 and 2). After electropolymerization of arginine along with template molecules, metal ions were extracted by CV runs in reverse potential range (+2.5 to −2.0 V) for five cycles (scan rate: 100 mV s−1) in 10 mL phosphate buffer solution, resulting in the fabrication of an imprinted MWCNT–polyarginine nanowire (Scheme 1). The non-imprinted nanowire-modified PGE was also prepared in a similar way, but in the absence of template molecules.
 |
| | Scheme 1 The schematic representation of the electrode fabrication. | |
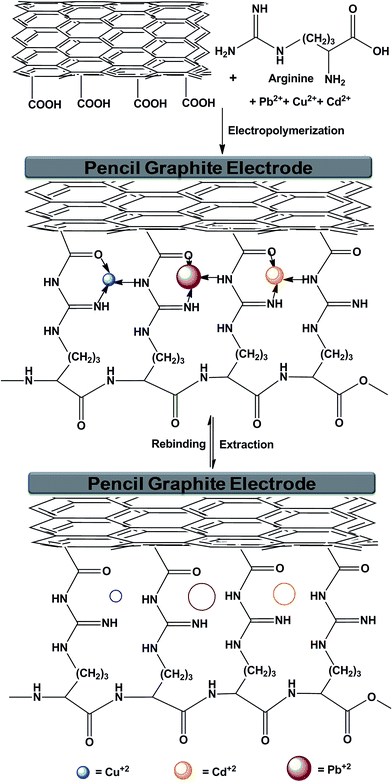 |
| | Scheme 2 Graphical representation of the binding mechanism between MWCNTs, polyarginine and the templates (Cu, Cd and Pb ions). | |
Sample preparation
Five representative samples from soil, water, fruit and/or vegetables and their corresponding plant leaves are collected from different populated areas, such as an industrial area, near a coal-mine, a river side, hills and our institute. Fruit and vegetable samples are either purchased commercially or donated unconditionally by the manufacturers. They were kept and transported in disposable metal free containers. For soil, fruit and vegetables, and their corresponding plant leaves, 15.0 g of each sample was weighed and placed into quartz mortar. Then, 10.0 mL of concentrated H2SO4 was added and evaporated to near dryness. Under heating conditions, concentrated hydrogen peroxide was added dropwise until the solution gets clear and evaporated. To this solution, water was added and heated to remove the unused hydrogen peroxide. The residue is then cooled and transferred into a 100 mL volumetric flask and diluted to the mark with water. For water samples, they were taken and used directly, as received, but were only filtered once to remove any solid impurities, if present.
Simulated digestion
Weighted food portions (such as mushrooms, cashew nuts, sweet potatoes, hazelnuts, soybeans, and raisins) were subjected to a simulated digestion procedure. Briefly, the maximum weight of 0.3 g of the homogenous analyte samples was in each case suspended in 2 mL 36% hydrochloric acid. Pepsin from porcine gastric mucosa was added at a ratio of 1/30, relative to the analyte weight. Test tubes were placed in a water bath shaker and incubated at 37 °C for 3 h. Afterwards, 4 mL sodium bicarbonate saturated solution was added to ensure alkaline pH. Pancreatin was added at a ratio of 1/30, relative to the analyte weight, and the incubation was continued for another 2 h in 37 °C. The enzymes were heat-inactivated through 10 min incubation in a water bath set at 100 °C. The extract was centrifuged at 6000 rpm for 10 min. The undigested residue was discarded.
Serum sample collection and preparation
Blood samples, 2 mL each, were collected from six randomly chosen, healthy students of our university, aged 20–25. They gave their written consent to take part in this study. All participants were fit; their BMI was normal, i.e. within the range of 20–25. The blood collection was carried out via venous puncture in a sterile environment at our institute health center. The serum was prepared by centrifuging the blood samples at 5000 rpm for 5 min. Serum samples, 1 mL each, were subjected to mineralization with 5 mL 65% nitric acid in a microwave digestor until completely decomposed.
Voltammetric analysis
For the electrochemical analysis, cadmium, copper and lead ions were pre-concentrated at the modified PGE surface at −0.5 V (accumulation potential) for 15 s (accumulation time) in a 0.03 M phosphate buffer solution. DPV in stripping mode (DPSV) was applied for quantitative analysis of metal ions in a potential range from +0.4 to −1.2 V, with a scan rate of 10 mV s−1, pulse amplitude of 25 mV, and pulse width of 50 ms. The simultaneous and selective determination of Cd(II), Cu(II) and Pb(II) has been performed under the same experimental conditions. All DPSV runs for each concentration of test analyte were quantified using the method of standard addition. The limit of detection (LOD) was calculated by three times the standard deviation from the blank measurement divided by the slope of the calibration plot of three different templates vs. the DPSV current.26
Results and discussions
Computational analysis of the monomer–template complex
The synthesis of a stable monomer–template complex is the foremost step in molecular imprinting. The stability of a complex depends on the interaction energy between the template and the monomer. Herein, we have studied the interaction energy between different amino acids (lysine, alanine, glutamic acid, histidine, cysteine, arginine and tyrosine) and metal ions (Table S1, ESI†) in gaseous phase using a density functional theory (DFT) method by utilizing hybrid Becke three-parameter exchange–correlation functional (B3LYP) and Lanl2DZ basis set (for metal ions). It was found that arginine shows the maximum interaction energy with metal ions.
In this work, MWCNT based nanowires also participated in the binding with metal ions, so their incorporation in the monomer–template complex is necessary. But the computational analysis of such a large complex is very time consuming and a cumbersome process. To resolve this problem and make the computational analysis less time consuming, herein, we have applied a new approach, namely a multi-layer ONIOM method for the calculation of the interaction energy between the monomer and the template along with MWCNTs. A comparative table between the Hartree–Fock method with a Lanl2DZ basis set (for metal ions) and the ONIOM method is portrayed in Table S2,† including the time it has taken to do the calculations. As shown in Table S2,† their energies are almost similar but the time taken for the calculation is much lower in the ONIOM method. The optimized geometry of each complex along with the MWCNTs are shown in Fig. 1A–C. The speciality of the ONIOM method can be understood by the fact that one can select different atoms in different layers and optimize them with a different level of theory. Herein, all the atoms presented in the monomer–template complex were optimized at a lower level of theory (semi-empirical, AM1) but the atoms involved in the bonding were optimized with the HF method (with the Lanl2DZ basis set). To explore the binding interaction between the templates and the monomer, a UV study was also performed (ESI, Text S1, Fig. S1†). The spectra for the reaction mixture are shown in Fig. S1A–C.† The results showed that the addition of metal ions, such as Cd2+, Cu2+ and Pb2+, caused apparent spectral changes due to the strong interaction between arginine and the metal cation.
 |
| | Fig. 1 Optimized geometry for the arginine–metal ion complex with a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using HF (A–C) and ONIOM (D–F) methods. 1 using HF (A–C) and ONIOM (D–F) methods. | |
Electropolymerization of arginine at the MWCNT modified PGE
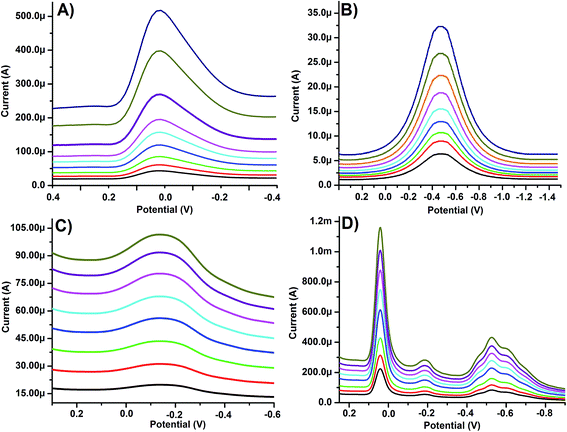
Arginine provides a broad and irreversible oxidation peak at −50 mV on PGE in a 0.03 M phosphate buffer solution (Fig. 2A and B). No cathodic peak was observed on the reverse scan. According to the literature, the electron oxidation of the amino group turns it into its corresponding cation radical,27 which forms carbon–nitrogen linkages at the carbon electrode surface.28 The current increases with each potential cycle, indicating the formation of the electro-active polymer film on the surface of the PGE (Fig. 2A and B). During the electrode fabrication, first of all, MWCNTs were coated onto the PGE surface, which is assumed to enhance the electrochemical properties of PGE. To confirm this, a CV analysis was performed. As shown in Fig. 2A, the current for arginine is in the order of 10−3 A, which gets ten times higher after coating with MWCNTs (Fig. 2B). This study showed that the MWCNTs do not only improve the electrochemical surface area, but also improve the sensitivity of the PGE. For the imprinted electrode fabrication, arginine along with the metal ions (Cu, Cd and Pb) were electropolymerized onto the MWCNT modified PGE. The corresponding CV runs are shown in Fig. 2C. Some clear reduction peaks are shown in the CV run, confirming the presence of the metal ions, which disappear after extraction in the reverse scan (Fig. 2D). This suggests the incorporation of metal ions in the polyarginine matrix during the polymerization step and the removal in the extraction step (Fig. 2C and D).
 |
| | Fig. 2 Cyclic voltammograms for the electropolymerization of arginine on (A) bare and (B) MWCNT modified PGE in the absence of metal ions. (C) Cyclic voltammograms for the electropolymerization of arginine on MWCNT modified PGE with the templates, and (D) the removal of the metal ions from the polymer film. | |
Morphological (SEM/EDAX) study
For the morphological study of modified and bare electrodes, SEM and EDAX analyses were conducted. In Fig. 3A, the SEM image of the bare PGE surface is shown, which is smooth and homogeneous. After modification, the carbon nanotubes are equally dispersed throughout the electrode surface which is clearly visible (Fig. 3B). One more information obtained from the SEM images is that both the adduct polymer (polymer having the template inside) and the non-imprinted polymer (NIP, polymer prepared without the template) have a very compact and smooth surface (Fig. 3C and E), whereas after template extraction, a wire like structure is visible on the MIP modified electrode surface (Fig. 3D). The clear difference in MIP and adduct modified electrode surfaces is shown in Fig. 3F, where one half represents the MIP modified electrode surface and the other half shows the morphology of the adduct modified electrode surface.
 |
| | Fig. 3 SEM images of (A) bare, (B) MWCNT modified, (C) adduct (MWCNT–polyarginine–template) modified, (D) imprinted (MWCNT–polyarginine–without template) modified, and (E) non-imprinted nanowire modified PGEs. (F) SEM image of a combined adduct and MIP modified PGE surface. | |
In order to confirm the presence of the Cu2+, Cd2+ and Pb2+ ions in the polymer matrix, EDAX spectra of the adduct (Fig. 4A) and the MIP (Fig. 4B) were recorded. The peaks corresponding to the metal ions are absent in the spectra of the MIP modified electrode surface, which suggests the complete extraction of the metal ions from the adduct polymer.
 |
| | Fig. 4 EDAX spectra of (A) adduct and (B) MIP–MWCNT–polyarginine modified PGEs. | |
Individual determination of metal ions
Under the optimized experimental conditions (accumulation potential, −0.5 V; accumulation time, 15 s), the estimation of the metal ions was performed in aqueous medium (optimization parameters are discussed in details in the ESI, Text S2, Fig. S2†). Herein, Fig. 5A–C show the DPSV response of the imprinted nanowire modified PGE towards Cd(II), Cu(II) and Pb(II) at different concentrations. Individually, Cd(II), Cu(II) and Pb(II) can be identified at potentials of 0.00, −0.50, and −0.20 V, respectively. According to the literature, the values found in the experiment are more negative than the standard ones. This may be due to the strong binding interaction between the polymer and the metal ion, which required more negative potential for the reduction of the respective metal ions. The DPSV current response of the imprinted nanowire modified PGE for the individual analysis of Cd(II), Cu(II) and Pb(II) was linear over a concentration range from 4.16 to 205.92 μg L−1, 9.54 to 471.46 μg L−1, and 6.69 to 204.16 μg L−1, respectively. The linear calibration equations for each metal ion are given below:
| (a) Cd(II): Ip (μA) = (4.813 ± 0.029)C (μg L−1) + (0.026 ± 0.495), n = 15, R2 = 0.99, LOD = 1.03 μg L−1. |
| (b) Cu(II): Ip (μA) = (0.463 ± 0.004)C (μg L−1) + (3.035 ± 1.016), n = 15, R2 = 0.99, LOD = 2.12 μg L−1. |
| (c) Pb(II): Ip (μA) = (0.883 ± 0.017)C (μg L−1) + (0.377 ± 1.67), n = 15, R2 = 0.99, LOD = 1.62 μg L−1. |
 |
| | Fig. 5 Differential pulse stripping voltammograms for the individual detection of (A) cadmium (μg L−1): 4.16, 6.17, 9.90, 11.83, 15.58, 19.32, 27.00, 39.47, 52.00; (B) copper (μg L−1): 9.54, 15.04, 20.44, 25.84, 31.24, 36.64, 42.04, 47.44, 57.15; (C) lead (μg L−1): 6.69, 11.12, 15.76, 20.41, 24.82, 29.47, 33.77, 37.41, and (D) the simultaneous detection of cadmium (μg L−1): 34.89, 49.13, 67.13, 95.46, 116.26, 138.03, 156.90, 182.44; copper (μg L−1): 95.56, 140.87, 192.90, 278.97, 340.96, 401.43, 447.65, 471.46; and lead (μg L−1): 6.69, 11.46, 17.33, 22.10, 26.86, 31.62, 36.49, 43.62 at imprinted nanowire modified PGEs at optimized parameters. | |
To validate the proposed method, the obtained results were compared with an earlier reported method.29 For this, an F-test and a t-test were conducted and the calculated values are given in Table S3.† It was observed that both the calculated values for the F-test and the t-test are lower than the theoretical values. This indicates that there was no significant difference between the proposed and earlier reported method. The reproducibility, repeatability and storage stability of the imprinted sensor was also performed and discussed in the ESI (Section S3†). A comparative study for the heavy metal detection in real samples was also conducted with a graphite furnace atomic absorption spectrometer (AAS) and the proposed method. The data obtained from both methods are portrayed in Table S4.† In the table it can be clearly observed that the results obtained from the proposed method is almost the same as provided using the AAS. This confirms the practical utility of the proposed method for heavy metal ion detection in real samples.
Simultaneous determination of Cd(II), Cu(II) and Pb(II)
To investigate the sensitivity of PGE and the intermolecular effects between the Cd, Cu and Pb ions, three different experiments were carried out under optimum conditions (pH 7.0). In each experiment, the concentration of one of the three compounds was changed while the concentration of the others were left constant. The reductive peak currents for the Cd, Cu and Pb ions increased linearly with an increase in their respective concentrations without affecting the other peak currents (Fig. 5D). The reduction peak potentials of the Cd, Cu and Pb ions on the modified electrode were separated completely into three well-defined peaks at 0.00, −0.56 and −0.20 V vs. Ag/AgCl, respectively (Fig. 5D). So, the simultaneous or selective detection of these metal ions using the imprinted nanowire modified PGE is feasible. These results confirm that the reduction processes of these metal ions at multi-imprinted nanowire modified PGE are independent from each other. Therefore, the proposed sensor can be used for real sample analysis.
Interference study
On the basis of individual and simultaneous detection, mutual interferences between the three heavy metals at the imprinted and non-imprinted nanowire modified PGEs were investigated. For a further study of the effect of interfering compounds on the metal ion detection, other foreign substances, such as ascorbic acid, glucose, iron, mercury, zinc and mixtures of these, were added to the standard solution. Both imprinted and non-imprinted electrodes show a small DPSV current for these interfering compounds, when studied individually. This is due to non-specific binding of the analyte or adsorption to the modified electrode surface, which can be easily curtailed by simple water washing. As predicted, after water washing, no detectable current was obtained on both imprinted and non-imprinted electrodes. However, in the presence of the interfering compounds, the imprinted nanowire modified PGE shows 100% current response for the template ions. As shown in Table 1, it may be concluded that the recognition sites, formed after the template extraction in the polymeric thin film, have the capability to distinguish the target metal ions through their size and shape. The imprinted nanowire modified PGE also shows 88–99% recoveries (RSD 1–5%) in the presence of the interfering compounds for the templates (metal ions), which accounts for the selectivity of the electrode (Table 1). So, from this study, we conclude that other compounds are completely not interfering with the metal ion estimation.
Table 1 Effects of the interfering compounds on the determination of Cd(II), Cu(II) and Pb(II)
| S. no. |
Name |
Concentration of analyte |
Recovery (%) |
RSD (%) |
| Added (μg L−1) |
Determined (μg L−1) |
| 1 |
Ascorbic acid |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
36.00 |
95 |
1.0 |
| (b) |
Spike copper |
45.00 |
44.60 |
99 |
4.0 |
| (c) |
Spike lead |
7.92 |
7.01 |
89 |
1.2 |
| 2 |
Aspartic acid |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
36.8 |
99 |
1.0 |
| (b) |
Spike copper |
45.00 |
44.6 |
99 |
1.2 |
| (c) |
Spike lead |
7.92 |
7.00 |
88 |
2.0 |
| 3 |
Mercury |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
34.00 |
91 |
2.34 |
| (b) |
Spike copper |
45.00 |
42.00 |
93 |
3.00 |
| (c) |
Spike lead |
7.92 |
7.00 |
88 |
1.00 |
| 4 |
Zinc |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
34.00 |
91 |
4.00 |
| (b) |
Spike copper |
45.00 |
42.50 |
94 |
5.00 |
| (c) |
Spike lead |
7.92 |
7.04 |
88 |
2.13 |
| 5 |
Lysine |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
35.00 |
94 |
3.13 |
| (b) |
Spike copper |
45.00 |
43.50 |
97 |
3.00 |
| (c) |
Spike lead |
7.92 |
7.19 |
90 |
2.00 |
| 6 |
Mix |
40.00 |
— |
— |
— |
| (a) |
Spike cadmium |
37.00 |
36.00 |
95 |
3.00 |
| (b) |
Spike copper |
45.00 |
44.00 |
97 |
2.00 |
| (c) |
Spike lead |
7.92 |
7.13 |
90 |
5.00 |
Total uptake of food-borne Cu(II), Cd(II), and Pb(II) ions in the human body
Copper is taken in as an essential element by the human body and has a good effect, if it is present under a certain limit. Contrary to copper, an excessive presence of Cd and Pb in foods is not desired.30 They have a fixed ‘Tolerable Upper Intake Level’ (UL) for our body, which is defined as the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects. To study the total uptake of these metal ions by normal food diet, blood samples of six people were tested (Table 2). Among six people, two drink more than five soft drinks a day, two have a cigarette smoking habit and two don’t have such a type of habit and eat a normal diet. The results portrayed in Table 2 show almost an equal distribution of copper in each sample. Whereas, cadmium is higher in people who smoke and lead is higher in people having more than five soft drinks a day. The numbers depicted in Table 2 correspond to the total amount of metal ions introduced into the human system. Based on the “Lead and copper rule (LCR)” of the U.S. Environmental Protection Agency (EPA), the action level for lead is 0.15 mg L−1 and 1.3 mg L−1 for copper.31 Whereas, for cadmium the tolerance limit was set to 3.0 mg L−1 in drinking water by the World Health Organization (WHO).32,33 This means that the average uptake in our study is at least 5-times lower than the UL and thereby considered safe for the persons having a normal diet as part of their daily routine. But the cadmium and lead uptake is high in some cases (soft drink drinkers and smokers), which is not considered to be a safe zone.
Table 2 Determination of metal ions in the blood samples of six people with imprinted nanowire modified PGE
| S. no. |
Type of daily diet |
Concentration of cadmium (μg) |
Concentration of copper (μg) |
Concentration of lead (μg) |
| Added |
Determined |
Recovery (%) |
RSD (%) |
Added |
Determined |
Recovery (%) |
RSD (%) |
Added |
Determined |
Recovery (%) |
RSD (%) |
| 1 |
Normal |
— |
0.01 |
— |
0.8 |
— |
1.00 |
— |
1.1 |
— |
0.10 |
— |
1.2 |
| 0.10 |
0.11 |
100.0 |
1.0 |
0.50 |
1.49 |
98.0 |
1.2 |
0.10 |
0.20 |
100.0 |
2.3 |
| 2 |
Normal |
— |
0.02 |
— |
0.9 |
— |
1.10 |
— |
1.6 |
— |
0.05 |
— |
1.5 |
| 0.10 |
0.12 |
100.0 |
1.1 |
0.50 |
1.62 |
104.0 |
1.8 |
0.10 |
0.15 |
100.0 |
2.0 |
| 3 |
Soft drink |
— |
0.04 |
— |
1.0 |
— |
1.50 |
— |
1.9 |
— |
0.25 |
— |
1.6 |
| 0.10 |
0.139 |
99.0 |
1.4 |
0.50 |
1.99 |
98.0 |
1.6 |
0.10 |
0.34 |
90.0 |
1.3 |
| 4 |
Soft drink |
— |
0.045 |
— |
1.5 |
— |
1.40 |
— |
2.1 |
— |
0.35 |
— |
1.8 |
| 0.10 |
0.144 |
99.0 |
1.8 |
0.50 |
1.90 |
100.0 |
1.9 |
0.10 |
0.45 |
100.0 |
1.4 |
| 5 |
Frequent smoker |
— |
0.10 |
— |
1.9 |
— |
2.00 |
— |
1.1 |
— |
0.15 |
— |
1.9 |
| 0.10 |
0.19 |
90.0 |
2.0 |
0.50 |
2.49 |
98.0 |
2.2 |
0.10 |
0.25 |
100.0 |
1.2 |
| 6 |
Frequent smoker |
— |
0.21 |
— |
1.6 |
— |
1.89 |
— |
1.4 |
— |
0.18 |
— |
1.0 |
| 0.10 |
0.31 |
100.0 |
1.0 |
0.50 |
2.40 |
102.0 |
1.8 |
0.10 |
0.28 |
100.0 |
2.0 |
Effect of metal ion accumulation in our biota
To explore the effect of metal ion accumulation in our environment, five different types of samples were studied, i.e. soil, water, fruit and vegetables, and their corresponding plant leaves. They were collected from different areas, viz. industrial area, coal-mine area, river side, hills and our institute (Table 3). We have first studied the metal ion accumulation in soil and tap water of each area and found that the concentration is very high in the industrial area and the contamination order is as follows: industrial area > coal-mine area > river side > hill area > normal populated area (our institute). The higher the concentration of the metal ions in the soil and water of a particular area, the higher the probability will be in the corresponding plants and vegetables. As predicted, the order of the metal ion concentration is similar in the fruits and/or vegetables, as well as their plant leaves (Table 3). Moreover, from this study, it also observed that a high level of metal ion is detected in the fruit and vegetable samples grown on highly contaminated soil, immigrated with contaminated water, as compared to those grown in low contaminated soil. From this study, we can conclude that metal ion accumulation in soil or water is more harmful as they are directly involved in our food chain.
Table 3 Estimation of the copper, cadmium, and lead content in different components of the biota
| S. no. |
Type of sample |
Area |
Coppera (mg) |
Cadmiuma (mg) |
Leada (mg) |
| Mean |
RSDb |
Mean |
RSDb |
Mean |
RSDb |
| Metal ion content is determined per 100 g mass or per 100 mL water. Relative standard deviation for three measurements. |
| 1 |
Soil |
Normal area |
0.216 |
2.1 |
0.40 |
1.2 |
0.025 |
2.5 |
| Industrial area |
1.21 |
2.1 |
2.6 |
2.3 |
0.15 |
3.0 |
| Coal-mine area |
1.32 |
1.3 |
2.8 |
2.0 |
0.16 |
1.2 |
| Hill area |
0.189 |
1.1 |
0.56 |
1.5 |
0.037 |
1.2 |
| River side area |
0.98 |
1.6 |
0.82 |
2.0 |
0.075 |
1.5 |
| 2 |
Water |
Normal area |
0.13 |
2.2 |
— |
— |
— |
— |
| Industrial area |
1.30 |
1.4 |
2.53 |
1.5 |
0.14 |
1.2 |
| Coal-mine area |
1.45 |
2.0 |
2.79 |
1.5 |
0.13 |
2.5 |
| Hill area |
0.26 |
2.1 |
0.42 |
3.0 |
0.031 |
2.0 |
| River side area |
0.86 |
1.8 |
0.74 |
2.5 |
0.065 |
1.5 |
| 3 |
Plant leaf (potato) |
Normal area |
0.15 |
1.3 |
0.41 |
1.5 |
0.020 |
2.0 |
| Industrial area |
1.12 |
1.5 |
2.42 |
2.0 |
0.14 |
2.0 |
| Coal-mine area |
1.30 |
2.2 |
2.45 |
2.0 |
0.15 |
2.0 |
| Hill area |
0.19 |
1.7 |
0.46 |
1.5 |
0.038 |
1.5 |
| River side area |
0.81 |
1.3 |
0.84 |
2.0 |
0.076 |
2.0 |
| 4 |
Plant leaf (tomato) |
Normal area |
0.14 |
2.1 |
0.39 |
2.5 |
0.021 |
2.5 |
| Industrial area |
1.19 |
1.8 |
2.32 |
1.5 |
0.17 |
1.5 |
| Coal-mine area |
1.28 |
1.6 |
2.21 |
2.0 |
0.14 |
2.0 |
| Hill area |
0.18 |
1.0 |
0.39 |
2.0 |
0.036 |
2.0 |
| River side area |
0.86 |
1.7 |
0.81 |
1.5 |
0.074 |
1.5 |
| 5 |
Plant leaf (mango) |
Normal area |
0.13 |
1.0 |
0.38 |
2.5 |
0.023 |
2.5 |
| Industrial area |
1.21 |
2.3 |
2.32 |
3.0 |
0.16 |
3.0 |
| Coal-mine area |
1.41 |
1.6 |
2.12 |
1.2 |
0.18 |
1.2 |
| Hill area |
0.17 |
1.1 |
0.35 |
1.2 |
0.029 |
1.2 |
| River side area |
0.78 |
1.5 |
0.74 |
1.5 |
0.065 |
1.5 |
| 6 |
Fruit/vegetable (potato) |
Normal area |
0.18 |
1.3 |
0.36 |
1.2 |
0.024 |
1.2 |
| Industrial area |
1.16 |
1.1 |
2.12 |
1.2 |
0.18 |
1.2 |
| Coal-mine area |
1.45 |
2.0 |
2.15 |
2.5 |
0.16 |
2.5 |
| Hill area |
0.21 |
2.0 |
0.36 |
2.0 |
0.031 |
2.0 |
| River side area |
0.89 |
1.4 |
0.71 |
1.5 |
0.069 |
1.5 |
| 7 |
Fruit/vegetable (tomato) |
Normal area |
0.18 |
1.3 |
0.34 |
2.0 |
0.025 |
2.0 |
| Industrial area |
1.13 |
1.5 |
2.29 |
2.0 |
0.15 |
2.0 |
| Coal-mine area |
1.28 |
2.1 |
2.28 |
2.0 |
0.19 |
2.0 |
| Hill area |
0.18 |
1.6 |
0.36 |
1.5 |
0.032 |
1.5 |
| River side area |
0.76 |
1.1 |
0.74 |
2.0 |
0.061 |
2.0 |
| 8 |
Fruit/vegetable (mango) |
Normal area |
0.14 |
2.0 |
0.34 |
2.5 |
0.019 |
2.5 |
| Industrial area |
1.02 |
1.7 |
2.30 |
1.5 |
0.19 |
1.5 |
| Coal-mine area |
1.24 |
2.3 |
2.25 |
2.0 |
0.11 |
2.0 |
| Hill area |
0.18 |
1.6 |
0.34 |
2.0 |
0.023 |
2.0 |
| River side area |
0.73 |
1.1 |
0.71 |
1.5 |
0.058 |
1.5 |
How much heavy metal are we taking from food?
Generally, cadmium and lead are not an active ingredient of our daily foods or diet. Therefore, their accumulation in a normal area is not so frequent. But copper is involved in our daily life, so its daily intake should be monitored. In normal circumstances, only a part of an incoming amount of metal ions per day is processed in the gastrointestinal tract. The largest dose of the consumed food is excreted and lost, together with potentially viable nutrients. To have an insight into the proportions of this process, we closely analyzed a series of food samples. It was our intention to analyze products that exhibit different characteristics of composition and a various extent of work is involved in their manufacturing process. We picked from an array of so called copper-rich products, such as mushrooms, cashew nuts, sweet potatoes, hazelnuts, soybeans, and raisins (Table 4). They were subjected to a simulated digestion study, which is thought to mimic processes that occur in the human gastrointestinal tract. This gave us an idea about the rate at which copper is released from foods, when subjected to metabolic conditions. We were willing to find out what the interplay is between the consumed amount of copper and its part that successfully undergoes digestion. The latter would now be free from the matrix and potentially bioavailable for absorption. As represented in Table 4, the highest release rate belongs to the nut family, where a 95% release rate was observed. However, the release rate of copper by other foods after digestion is found to be in the range of 75–90%. From this study, it is clearly observed that some foods, which are low in metal content, can release more metal ions based on their digestion during food processing inside our gut.
Table 4 Content of copper determined in edible plant product samples before and after simulated digestion
| S. no. |
Edible products |
Total copper content [mg per 100 g] |
Copper after digestion [mg per 100 g] |
Release rate [%] |
| Meana |
RSDb |
Meana |
RSDb |
| Mean of three replicate values. Relative standard deviation. |
| 1 |
Mushrooms |
3.00 |
1.4 |
2.70 |
1.2 |
90.00 |
| 2 |
Cashew nuts |
2.22 |
2.0 |
2.10 |
2.5 |
94.60 |
| 3 |
Sweet potato |
0.12 |
2.5 |
0.10 |
1.5 |
83.33 |
| 4 |
Hazelnuts |
1.75 |
1.5 |
1.63 |
2.0 |
93.14 |
| 5 |
Soybeans |
0.70 |
1.0 |
0.53 |
1.2 |
75.71 |
| 6 |
Raisins |
0.32 |
1.2 |
0.27 |
2.5 |
84.37 |
Conclusion
In this study, we examined the role of a multi-template imprinted nanowire modified PGE for trace level sensing of heavy metal ions. The proposed sensor was applied to various types of real samples. Herein, first, we have studied the impact of our daily life food intake, which is mostly responsible for the heavy metal ion risk in our health. Similarly, we have also studied that the products, i.e. fruit and vegetables, of plants, which grow in the presence of metal ion contaminated water and soil, are also affected by heavy metal accumulation. In another study, we have also explored the release rate of copper ion by different food samples, which suggests that whatever we eat is directly responsible of what we gain. At last we can conclude that a more conscious risk management in this field would allow for a less constraining diet and thereby more diverse nutrition.
Acknowledgements
Authors are thankful to the Department of Science and Technology, Government of India for sanction of Fast Track Research Project for Young Scientists to Dr Rashmi Madhuri (Ref. no. SB/FT/CS-155/2012) and Dr Prashant K. Sharma (Ref. no. SR/FTP/PS-157/2011). Dr Sharma (FRS/34/2012-2013/APH) and Dr Madhuri (FRS/43/2013-2014/AC) are also thankful to the Indian School of Mines, Dhanbad, for the grant of the Major Research Project under the Faculty Research Scheme. We are also thankful to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy, Government of India for the major research project. S.P. & E.R. are also thankful to the Indian School of Mines, Dhanbad, for the Junior Research Fellowship.
Notes and references
- A. Fairbrother, R. Wenstel, K. Sappington and W. Wood, Ecotoxicol. Environ. Saf., 2007, 68, 145–227 CrossRef CAS PubMed.
- Z. Z. Chen, L. Zhu and K. J. Wilkinson, Environ. Sci. Technol., 2010, 44, 3580–3586 CrossRef CAS PubMed.
- U. Borgmann, W. P. Norwood and D. G. Dixon, Hum. Ecol. Risk Assess., 2008, 14, 266–289 CrossRef CAS.
- M. Mirzaei, M. Behzadi, N. M. Abadi and A. Beizaei, J. Hazard. Mater., 2011, 186, 1739–1743 CrossRef CAS PubMed.
- R. K. Sharma, A. Puri, A. Kumar and A. Adholeya, J. Environ. Sci., 2013, 25, 1252–1261 CrossRef CAS.
- M. Faraji, Y. Yamini, A. Saleh, M. Rezaee, M. Ghambarian and R. Hassani, Anal. Chim. Acta, 2010, 659, 172–177 CrossRef CAS PubMed.
- D. Chen, B. Hu and C. Huang, Talanta, 2009, 78, 491–497 CrossRef CAS PubMed.
- C. Chen, X. Niu, Y. Chai, H. Zhao and M. Lan, Sens. Actuators, B, 2013, 178, 339–342 CrossRef CAS PubMed.
- S. Abbasi, K. Khodarahmiyana and F. Abbasi, Food Chem., 2011, 128, 254–257 CrossRef CAS PubMed.
- U. Divrikli, A. A. Kartal, M. Soylak and L. Elci, J. Hazard. Mater., 2007, 145, 459–464 CrossRef CAS PubMed.
- L. Cui, J. Wu, J. Li, Y. Ge and H. Ju, Biosens. Bioelectron., 2014, 55, 272–277 CrossRef CAS PubMed.
- L. Wu, X. Fu, H. Liu, J. Li and Y. Song, Anal. Chim. Acta, 2014, 851, 43–48 CrossRef CAS PubMed.
- M. Jarczewska, E. Kierzkowska, R. Ziółkowski, Ł. Górski and E. Malinowska, Bioelectrochemistry, 2015, 101, 35–41 CrossRef CAS PubMed.
- X.-C. Fu, J. Wu, L. Nie, C.-G. Xie, J.-H. Liu and X.-J. Huang, Anal. Chim. Acta, 2012, 720, 29–37 CrossRef CAS PubMed.
- T. Ndlovu, B. B. Mamba, S. Sampath, R. W. Krause and O. A. Arotiba, Electrochim. Acta, 2014, 128, 48–53 CrossRef CAS PubMed.
- B. Feier, D. Floner, C. Cristea, E. Bodoki, R. Sandulescu and F. Geneste, Talanta, 2012, 98, 152–156 CrossRef CAS PubMed.
- V. Beni, G. Collins and D. W. M. Arrigan, Anal. Chim. Acta, 2011, 699, 127–133 CrossRef CAS PubMed.
- R.-X. Xu, X.-Y. Yu, C. Gao, Y.-J. Jiang, D.-D. Han, J.-H. Liu and X.-J. Huang, Anal. Chim. Acta, 2013, 790, 31–38 CrossRef CAS PubMed.
- L. Zhu, L. Xu, B. Huang, N. Jia, L. Tan and S. Yao, Electrochim. Acta, 2014, 115, 471–477 CrossRef CAS PubMed.
- H. Huang, T. Chen, X. Liu and H. Ma, Anal. Chim. Acta, 2014 DOI:10.1016/j.aca.2014.09.010.
- H. Bagheri, A. Afkhami, H. Khoshsafar, M. Rezaei and A. Shirzadmehr, Sens. Actuators, B, 2013, 186, 451–460 CrossRef CAS PubMed.
- L. Xiao, H. Xu, S. Zhou, T. Song, H. Wang, S. Li, W. Gan and Q. Yuan, Electrochim. Acta, 2014, 143, 143–151 CrossRef CAS PubMed.
- B. B. Prasad, D. Jauhari and A. Verma, Talanta, 2014, 120, 398–407 CrossRef PubMed.
- D. Kumar, R. Madhuri, M. P. Tiwari, P. Sinha and B. B. Prasad, Adv. Mater. Lett., 2011, 2, 294–297 CrossRef CAS PubMed.
- M. Moyo, J. O. Okonkwo and N. M. Agyei, Enzyme Microb. Technol., 2014, 56, 28–34 CrossRef CAS PubMed.
- D. A. Skoog, F. T. Holler and T. A. Neiman, Principal of instrumental analysis, Hartcourt Brace college publishers, Orlando, 5th edn, 1998 Search PubMed.
- C. K. Mann, Anal. Chem., 1964, 36, 2424–2426 CrossRef CAS.
- L. Zhang and X. Lin, Analyst, 2001, 126, 367–370 RSC.
- Z. Wang, P. M. Lee and E. Liu, Thin Solid Films, 2013, 544, 341–347 CrossRef CAS PubMed.
- K.-C. Kim, Y.-B. Park, M.-J. Lee, J.-B. Kim, J.-W. Huh and D.-H. Kim, Food Res. Int., 2008, 41, 411–418 CrossRef CAS PubMed.
- EPA. “Maximum Contaminant Level Goals and National Primary Drinking Water Regulations for Lead and Copper; Final Rule.” Federal Register, 56 F.R. 26460, 1991-06-07. 40 CFR Part 141, Subpart I.
- WHO (World Health Organization), Fifty-third report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series 896, Geneva, Switzerland, 2000 Search PubMed.
- H. C. Mandalia, Health Sciences Research, 2014, 3, 30–34 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1, S2, S3, and S4; Section S1, S2 and S3; Fig. S1 and S2. See DOI: 10.1039/c4ra08875a |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n; template
n; template![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer, where n = 1, 2, and 3) were designed and interaction energies were calculated using the following equation, where n refers to the monomer number in the template–monomer complexes:
monomer, where n = 1, 2, and 3) were designed and interaction energies were calculated using the following equation, where n refers to the monomer number in the template–monomer complexes:

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using HF (A–C) and ONIOM (D–F) methods.
1 using HF (A–C) and ONIOM (D–F) methods.




