Photochromic Ag:TiO2 thin films on PET substrate
Abstract
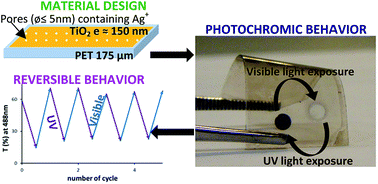
TiO2:Ag nanocomposite thin films were prepared on plastic PET substrate in two steps. First, a sol–gel route and evaporation induced self-assembly (EISA) method were used to fabricate the TiO2 matrix. The use of thermal treatment being discordant with the plastic substrate, the mesoporosity was realized by chemical extraction or infrared annealing. Silver was then introduced in the film porosity in the form of ions by soaking the films into a silver salt. A reversible photochromic behavior was successfully demonstrated after successive cycles of UV and visible laser exposures without degrading the PET substrate. This laser-induced colour changes are based on the reversible growth and dissociation of silver nanoparticles within the titania matrix.

 Please wait while we load your content...
Please wait while we load your content...