Tetraethylammonium iodide catalyzed synthesis of diaryl ketones via the merger of cleavage of C–C double bonds and recombination of aromatic groups†
Abstract
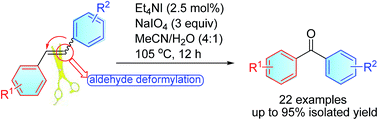
An efficient method for synthesizing diaryl ketones via merging of oxidative cleavage of C–C double bonds and recombination of aromatic groups is developed with Et4NI (2.5 mol%) as the catalyst and NaIO4 as the oxidant. The control experiments provide valuable mechanistic insights into the formation of diaryl ketones, and suggest that NaIO4 serves as an epoxidation and nucleophilic deformylation reagent.

 Please wait while we load your content...
Please wait while we load your content...