The role of ozone in the ozonation process of graphene oxide: oxidation or decomposition?†
Abstract
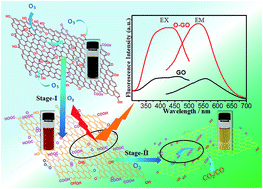
We took ozonation as an effective method to re-oxidize graphene oxide (GO) and discussed the behaviour of ozone in ozonation process according to the changes of optical properties, compositions and structures. The results indicate that the ozonation process may involve the oxidation stage and decomposition stage. The research would improve the understanding of the ozonation process and promote the future application of ozonized graphene oxide as the precursor.

 Please wait while we load your content...
Please wait while we load your content...