Tuning unexpected room temperature ferromagnetism in heteroepitaxial PbTiO3 thin films fabricated by hydrothermal epitaxy: crystal quality
Jing Zhang†
ab,
Zhaolong Yanga,
Fengzhen Lva,
Cunxu Gao†*a and
Desheng Xue*a
aKey Laboratory for Magnetism and Magnetic Materials of MOE, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: gaocunx@lzu.edu.cn; xueds@lzu.edu.cn; Fax: +86-0931-8914160; Tel: +86-0931-8912237
bCollege of Electric and Information Engineering, Beifang University of Nationalities, Yinchuan 750021, P. R. China
First published on 10th November 2014
Abstract
The unexpected room temperature ferromagnetism in well-crystallized lead titanate (PbTiO3) heteroepitaxial thin films is attributed to crystal quality, which is produced by mild hydrothermal epitaxy on strontium titanate (100) substrates. The morphological, structural and magnetic properties of these epitaxial films were examined by a variety of experimental techniques. In the growth process of PbTiO3 films with a perovskite structure, the nucleations appear as islands firstly and subsequent growth follows the layer-by-layer growth mode; simultaneously as increase of growth time the stress between films and substrates releases gradually, the lattices of films follow substrates at first and then the films obey their own lattices; the crystal quality is increasing during this growth process. Meanwhile the results of magnetic measurement reveal that our films have the unambiguous ferromagnetism, and the strength of the ferromagnetic component decreases monotonously as increasing crystal quality. In addition, the growth mechanism involved a dissolution–crystallization mechanism is exposed.
1 Introduction
The possibility of ferromagnetism (FM) in oxides has been widely debated since 2000.1 After that, a lot of theoretical and experimental works were carried out in order to find the promised FM behaviours.2–5 However, the obtained results were quite contradictory.6–9 The viewpoint that cation and/or anion defects in nano-scale oxides are the reason for FM became generally accepted in the past few years both theoretically and experimentally.5,10–14 In order to study further the relationship between FM and defects, a few groups focused on model systems with excellent structural and/or crystal quality: FM correlates with the presence of structural defects in epitaxial (Ti, Cr)O2 with excellent structural quality;15 high quality single-crystals and oxygen vacancy ferromagnetic ordered variant of (Y, Sr)CoO3 have been produced;16 the defects play important roles in determining the ferromagnetic characteristics of the (Zn, Co)S films through adjusting crystal quality.17 The above studied systems with high crystal and/or structural quality gave the new path to understand the unexpected FM; but owing to presence of ferromagnetic elements (Cr, Co, etc.), the impact from secondary phases such as clusters could not be excluded.Based on the above significant works, we chose epitaxial films without any ferromagnetic elements as our experimental carriers; considerable efforts have recently been made to understand the kinetic processes which control the nucleation and subsequent growth of submonolayer islands in various homoepitaxial and heteroepitaxial systems,18–23 so FM which was induced by crystal quality should be tuned though the experimental conditions. Understanding the physics of epitaxial growth has been a longstanding problem in surface physics and material science.24–26 The primary methods in obtaining well-crystallized epitaxial films are molecular beam epitaxy,27,28 plasma-assisted molecular beam epitaxy,29 chemical vapour deposition,18 pulsed laser deposition,30 and other related vapor-phase techniques.25 However, all the above techniques should require a high-temperature history (>500 °C) for crystallization during or after film formation. This can eventually result in deterioration of film quality due to inhomogeneous crystallization, selective evaporation or deposition, and even seriously undesired chemical reactions.31,32 Nevertheless, an important mild chemical route named hydrothermal method has been developed as a nontraditional way for growing epitaxial single-crystal thin films; that was called as hydrothermal epitaxy. Hydrothermal epitaxy is a low-temperature way to create homoepitaxial or heteroepitaxial thin films through utilization of the chemical reaction of inorganic materials on structurally similar substrates.33–35 Film growth occurs at substantially lower temperature than vapor methods mentioned above, which diminishes the problems of high processing temperature and the need for ultrahigh vacuum.36 And this novel synthetic technique offers the advantage of simple instrumentation, high purity, high homogeneity, and with no need of a post deposition annealing for crystallization.31,32
Ferroelectric thin films [perovskite structure such as BaTiO3, PbTiO3 (PTO), and Pb(Zr, Ti)O3] with epitaxial structure are preferable or essential for engineering devices including ultrasonic sensors, infrared detectors, and ferroelectric random access memories because it could potentially exhibit superior crystallographic and physical properties, resulting in improved device performance and characteristics.33,37,38 In these films, PTO is one of the fundamental ferroelectric materials and important as a component of solid solutions;39 while absence of ferromagnetic element gave us an opportunity to fill in the blank about unexpected FM. Further, PTO thin films have been studied by the hydrothermal epitaxy using a few variety of substrates, precursors (such as Pb(NO3)2, TiO2; Pb(OH)2, titanium substrate, etc.), processing temperatures, and mineralizers.31,33,39–41 More importantly, the ability to form hydrothermal PTO films depends strongly on the concentration of both KOH and Pb(OH)2, only in a limited concentration region well-crystallized films were obtained; the grain size and crystallinity was generally increased with the concentration of KOH in the starting solution, a reduction in the lead and titanium concentrations and the effective high alkali concentration enabled an increase in thickness of PTO films.39,41 However, there is few report of the relationship between FM and crystal quality in this ferroelectric system. In this letter, we demonstrated that well-crystallized PTO heteroepitaxial thin films on the SrTiO3 (STO) (100) substrate were produced by the mild hydrothermal epitaxy and tried to understand the growth process of epitaxial films; more importantly, the unexpected room temperature (RT) FM could be tuning by the crystal quality through changing the reaction time. In addition, we attempted to discuss the growth mechanism of epitaxial PTO films.
2 Experimental
2.1 Hydrothermal synthesis of PTO thin films
All the chemical reagents as starting materials are analytic grade and 5 × 5 × 0.5 mm3 STO(100) single crystal substrates were used without any further treatment [cubic, a0 = 3.905 Å, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (221), PDF no. 35-0734]. Typically, 1.0 g lead nitrate [Pb(NO3)2] and 0.2 g TiO2 powders were dissolved into deionizer water (50 mL), and then the solution was kept under magnetic stirring. After that, an alkaline aqueous solution of 10 mol L−1 KOH (15 mL) was added to the above solution under intensive stirring. The as-prepared mixed solution was moved into a Teflon-lined stainless steel autoclave of 100 mL capacity with the fixed STO substrate, and the autoclave was contained at 150 °C for 24 h. After the reaction, the PTO thin film was washed with deionizer water, and dried in air. Four different reaction times (3, 6, 12, and 24 h) were studied, while the corresponding specimens were denoted as PTO-3, PTO-6, PTO-12, and PTO-24.
m (221), PDF no. 35-0734]. Typically, 1.0 g lead nitrate [Pb(NO3)2] and 0.2 g TiO2 powders were dissolved into deionizer water (50 mL), and then the solution was kept under magnetic stirring. After that, an alkaline aqueous solution of 10 mol L−1 KOH (15 mL) was added to the above solution under intensive stirring. The as-prepared mixed solution was moved into a Teflon-lined stainless steel autoclave of 100 mL capacity with the fixed STO substrate, and the autoclave was contained at 150 °C for 24 h. After the reaction, the PTO thin film was washed with deionizer water, and dried in air. Four different reaction times (3, 6, 12, and 24 h) were studied, while the corresponding specimens were denoted as PTO-3, PTO-6, PTO-12, and PTO-24.
2.2 Characterization
Microstructural characterization of the thin films was performed with a scanning electron microscope (SEM, Hitachi S-4800, Chiyoda-ku, Japan). Phase identification and film orientation was determined by high-resolution X-ray diffractometry (HRXRD) (Bede D1, England) using longitudinal ω–2θ scans with an analyzer. Electron backscattering diffraction (EBSD) was carried out in a Zeiss Ultra-55 scanning electron microscope equipped with an EDAX-TSL EBSD system. The measurements of magnetic properties were made using Quantum Design MPMS magnetometer based on superconducting quantum interference device (SQUID, San Diego, CA, USA).3 Results and discussion
To study the growth process of PTO films, SEM images of the as-grown films were measured and some typical ones are shown in Fig. 1. After 3 h of growth, in Fig. 1a, the PTO thin film can completely cover the STO substrate, and presents well-aligned regular quasi-tetragonal cuboids. The cuboids exhibit the side-length of 100 ∼ 300 nm and an average height–length of 750 nm (the inset of Fig. 1a). The specimen removed from the solution after 6 h shows that growth initiates by quasi-tetragonal cuboids at STO substrate followed by coalescence to form two dimensional layers after longer periods as shown in Fig. 1b, and could achieve the full smooth coverage. With increasing of growth time, the quality of films becomes better, the average side-length in PTO-12 turns into 3 μm (Fig. 1c). Sequential growth experiment shows that PTO-24 in Fig. 1d becomes rectangular prisms from regular microflats. As a result, the nucleations appear firstly as islands; as the growth time increased, layers become to appear; rectangular prisms turn thicker and thicker as long as the layers have already covered totally; simultaneously crystal quality of the PTO films becomes better and better. Based on these results, the PTO thin films might grow epitaxially on the substrates.In order to confirm the epitaxial growth, the original phases and orientation of films and substrates were needed to be identified. The hydrothermally as-grown PTO films on the STO(100) substrates grown at various times were qualitatively assessed by symmetric HRXRD longitudinal ω–2θ scans, shown in Fig. 2, elucidating that the films are well aligned and have an epitaxial relationship with substrates. What's more, the pseudo-Kikuchi patterns obtained by EBSD (not shown here) were also used to further understand the crystallographic information of the films' process and exhibit that both the PTO films with high crystal quality and STO substrate have the very similar crystal structures and orientations. For each HRXRD curve, two peaks originating from STO substrates are clearly visible, meanwhile the peak from measurement background keeps located at about 38.2°. The reflection peaks measured by HRXRD patterns with ω–2θ scans continuous line originate mostly from the out-of-plane lattices of films. However, there are two points distinct and hard to understand at least: firstly, the observed peaks in our results seem to change PTO (h00) (h = 1, 2) into PTO (00l) (l = 1, 2) [P4/mmm (123), PDF no. 06-0452] in the different growth times; secondly, HRXRD patterns show usually sharper peaks as the crystal quality increases, which were not found in our samples. Therefore, lattice mismatch between PTO and STO was studied to solve these conundrums: in plane lattice mismatch was nearly close to zero (Δa/a0 ≈ 0.15%); meanwhile, out-of-plane lattice mismatch is 6.3%, much larger than that in plane one; this distinction elucidates that the strain between PTO films along the c-axis direction and STO substrate is small, the films should be inclined to have the clear out-of-plane orientation. Combining with the HRXRD patterns, the growth process of PTO films is as follow: firstly, the lattices of films follow STO substrates because of the larger stress, that results in formation of the similar lattices with STO substrates; and then as growth of films the stress releases gradually, the films start to grow along more stable out-of-plane direction; finally lattices in PTO's own appear and the HRXRD pattern has only (00l) peaks with clear c-axis orientation. In a word, stress is releasing gradually and crystal quality becomes higher as growth time is increased, which can be interpreted as decrease the defect concentration, crystal and/or grain boundaries and so on.
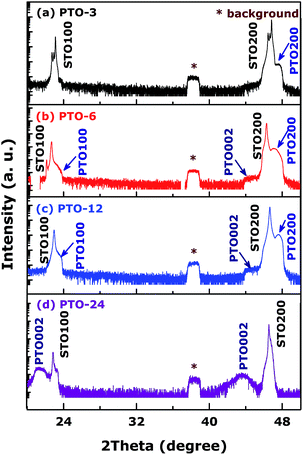 | ||
| Fig. 2 Symmetric HRXRD longitudinal ω–2θ scans of the PTO films on STO(100) substrates grown at various times. | ||
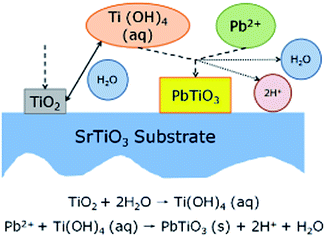
After studying the growth process of heteroepitaxial films, the kinetics process of hydrothermal crystallization of PTO should be studied further. However, that was blank study as far as we known; we were motivated to conjecture based on the results of similar epitaxial BaTiO3 films,31,32,42 shown in Fig. 3. TiO2 deposited firstly on the substrates. As the hydroxylation continues, a gel particle of Ti(OH)4 reactive intermediate which is absorbed on the surface of substrate will form. When the concentrations of Ti(OH)4 and Pb2+ in the environment reach their supersaturated values, they react with each other, and in the vicinity give rise to PTO precipitates. The heterogeneous nucleation of PTO, crystals on the substrate and subsequent growth from them occurs for a certain period of time. It could be concluded that the precise path of hydrothermal crystallisation is very sensitive to the choice of reagents' concentrations, especially the time of reaction in this paper.
Through the above analysis, the crystal quality of our films becomes better and better as increasing of growth time; those motivated us to carry out further the comparative study on the unexpected RT FM induced by structural and/or crystal quality reported in various oxides.31,37,43–48 In our system, when the growth time enhances, the films' quality increases, and the crystals become bigger and better in the process of crystal growth; that is, the FM in the PTO films would intend to weaken if that were induced by crystal quality. In order to confirm our speculate, the magnetization curves as a function of applied magnetic field (M–H) at 10 K and 300 K of PTO films were measured firstly, shown in Fig. 4, where the contributions of the diamagnetic (DM) signals were deducted. M–H curves indicate that the samples have the unambiguous FM; moreover, the shape of M–H curves of each sample taken at 10 K and 300 K is quite similar indicating that the samples are certainly in a ferromagnetic state over a wide range of temperature.38 And for the three different samples the saturation magnetization (Ms) decreases as the temperature increases and the variance of Ms is similar at about 18 ∼ 19%, which is a typical behavior of ferromagnetic materials;49 more importantly, Ms at 10 K or 300 K decreases monotonously with increase of the growth time, respectively. In order to further explore the magnetic properties, the temperature dependence of the magnetization (M–T) curve for PTO-3, because of its largest Ms, was measured in the range of 10 ∼ 300 K at the direct current field of 1000 Oe, and the result is shown in the inset of Fig. 4. It can be seen that the magnetization is stable with increasing temperature in the low-temperature region, followed by a gradual decline in the magnetization that does not reach zero until 300 K, indicating that the Curie temperature of this sample is higher than 300 K, which can be got by M–H curves. As the result, when the growth time increases, the Ms decreases monotonously, the crystal quality of our films inducing FM should enhances gradually; the results of microstructural characterization confirm this conclusion.
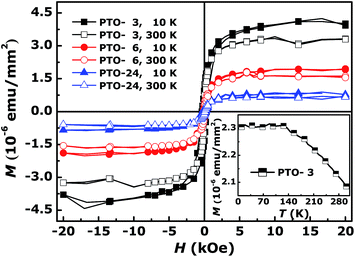 | ||
| Fig. 4 M–H curves of PTO-3, PTO-6, and PTO-24 at 10 K and 300 K (deducted DM signals). The inset is the M–T curve for PTO-3. | ||
The similar study in oxides on the relation between structural and/or crystal quality and ferromagnetism was reported, which involves various defects more or less. The magnetic properties might be related to the formation of acceptor-like defects (Oi and OZn) in the Zn0.8Co0.2O and Zn0.8Mn0.2O films; in addition, the higher ratio of grain-boundary area to grain volume is the key roles of grain boundaries and acceptors with respect to FM properties.48 The changes in the structure and contiguity of a ferromagnetic grain boundary foam are responsible for the magnetic properties of pure ZnO and Fe doped ZnO.47 In our system, stress is releasing gradually and crystal quality becomes higher as growth time is increased, while the good crystal quality means decrease of various defects' concentration necessarily, such as the defect concentration, crystal and/or grain boundaries, etc. At the same times, appearance of FM can be induced by various defects, and FM decreases as the crystal quality becomes better through decreasing of defects probably.
4 Conclusions
Our work presented the direct experimental evidence for the heteroepitaxial stabilization of PTO films by the hydrothermal epitaxy method, which suggests the growth progress (nucleations as islands and subsequent layer-by-layer growth). All the films have the clear FM, and Ms decreases monotonously with the increase of crystal quality. Our work presented offers a new path to understand the relationship between crystal quality and the observed RT FM, which should be powerful and useful in the future search.Acknowledgements
This work was supported by National Basic Research Program of China (Grant no. 2012CB933101), National Natural Science Foundation of China (Grant nos 11274147, 51371093 and 11034004) and the Fundamental Research Funds for the Central Universities (nos lzujbky-2013-ct01 and lzujbky-2014-174).References
- T. Dietl, H. Ohno, F. Matsukura, J. Cibert and D. Ferrand, Science, 2000, 87, 1019–1022 CrossRef.
- N. H. Hong, J. Sakai and V. Brizé, J. Phys.: Condens. Matter, 2007, 19, 036219 CrossRef.
- D. Gao, D. Xue, Y. Xu, Z. Yan and Z. Zhang, Electrochim. Acta, 2009, 54, 2392–2395 CrossRef CAS PubMed.
- F.-Y. Ran, M. Subramanian, M. Tanemura, Y. Hayashi and T. Hihara, Appl. Phys. Lett., 2009, 95, 112111 CrossRef PubMed.
- C. D. Pemmaraju and S. Sanvito, Phys. Rev. Lett., 2005, 94, 217205 CrossRef.
- C. D. Pemmaraju and S. Sanvito, Phys. Rev. Lett., 2005, 94, 217205 CrossRef.
- R. K. Singhal, P. Kumari, A. Samariya, S. Kumar, S. C. Sharma, Y. T. Xing and E. B. Saitovitch, Appl. Phys. Lett., 2010, 97, 172503 CrossRef PubMed.
- S. W. Fan, K. L. Yao and Z. L. Liu, Appl. Phys. Lett., 2009, 94, 152506 CrossRef PubMed.
- R. P. Panguluri, P. Kharel, C. Sudakar, R. Naik, R. Suryanarayanan, V. M. Naik, A. G. Petukhov, B. Nadgorny and G. Lawes, Phys. Rev. Lett., 2005, 95, 217203 CrossRef.
- Y. Li, R. Deng, B. Yao, G. Xing, D. Wang and T. Wu, Appl. Phys. Lett., 2010, 97, 102506 CrossRef PubMed.
- N. N. Bao, J. B. Yi, H. M. Fan, X. B. Qin, P. Zhang, B. Y. Wang, J. Ding and S. Li, Scr. Mater., 2012, 66, 821–824 CrossRef CAS PubMed.
- D. Kim, J. Hong, Y. R. Park and K. J. Kim, J. Phys.: Condens. Matter, 2009, 21, 195405 CrossRef PubMed.
- M. S. Si and D. S. Xue, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 193409 CrossRef.
- T. L. Makarova, B. Sundqvist, R. Hö hne, P. Esquinazi, Y. Kopelevich, P. Scharff, V. A. Davydov, L. S. Kashevarova and A. V. Rakhmanina, Nature, 2001, 716–718 CrossRef CAS PubMed.
- T. C. Kaspar, S. M. Heald, C. M. Wang, J. D. Bryan, T. Droubay, V. Shutthanandan, S. Thevuthasan, D. E. McCready, A. J. Kellock, D. R. Gamelin and S. Chambers, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 165208 CrossRef.
- C. L. Fleck, G. Balakrishnan and M. R. Lees, J. Mater. Chem., 2011, 21, 1212–1217 RSC.
- W.-S. Ni and Y.-J. Lin, J. Appl. Phys., 2012, 112, 063712 CrossRef PubMed.
- L. J. Lauhon, M. S. Gudiksen, D. Wang and C. M. Lieber, Nature, 2002, 420, 57–61 CrossRef CAS PubMed.
- L. Gao, J. R. Guest and N. P. Guisinger, Nano Lett., 2010, 10, 3512–3516 CrossRef CAS PubMed.
- C. Coletti, C. Riedl, D. S. Lee, B. Krauss, L. Patthey, K. von Klitzing, J. H. Smet and U. Starke, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 235401 CrossRef.
- C. Riedl, C. Coletti, T. Iwasaki, A. A. Zakharov and U. Starke, Phys. Rev. Lett., 2009, 103, 246804 CrossRef CAS.
- R. Shimizu, K. Iwaya, T. Ohsawa, S. Shiraki, T. Hasegawa, T. Hashizume and T. Hitosugi, ACS Nano, 2011, 5, 7967–7971 CrossRef CAS PubMed.
- G. Koster, B. L. Kropman, G. J. H. M. Rijnders, D. H. A. Blank and H. Rogalla, Appl. Phys. Lett., 1998, 73, 2920–2922 CrossRef CAS PubMed.
- J. G. Amar and F. Family, Phys. Rev. Lett., 1995, 74, 2066–2069 CrossRef CAS.
- M. Copel, M. C. Reuter, E. Kaxiras and R. M. Tromp, Phys. Rev. Lett., 1989, 63, 632–635 CrossRef CAS.
- J. Palisaitis and R. Vasiliauskas, Physics of Advanced Materials Winter School, 2008, 1–16.
- S. C. Erwin, C. Gao, C. Roder, J. Lähnemann and O. Brandt, Phys. Rev. Lett., 2011, 107, 026102 CrossRef.
- C. Gao, H.-P. Schönherr and O. Brandt, Appl. Phys. Lett., 2010, 97, 031906 CrossRef PubMed.
- P. Dogan, O. Brandt, C. Pfüller, J. Lähnemann, U. Jahn, C. Roder, A. Trampert, L. Geelhaar and H. Riechert, Cryst. Growth Des., 2011, 11, 4257–4260 CAS.
- J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Schlom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M. Wuttig and R. Ramesh, Science, 2003, 299, 1719–1722 CrossRef CAS PubMed.
- W. ping Xu, L. Zheng, H. Xin, C. Lin and M. Okuyama, J. Mater. Res., 1996, 11, 821–824 CrossRef.
- W.-P. Xu, L. Zheng, C. Lin and M. Okuyama, Philos. Mag. B, 1998, 77, 177–185 CrossRef CAS.
- J. H. Jeon and S. K. Choi, Appl. Phys. Lett., 2007, 91, 091916 CrossRef PubMed.
- F. Lv, J. Zhang, C. Gao, L. Ma, D. Gao, S. Zhou and D. Xue, Nanoscale Res. Lett., 2014, 9, 266 CrossRef PubMed.
- P. Zhang, C. Gao, F. Lv, Y. Wei, C. Dong, C. Jia, Q. Liu and D. Xue, Appl. Phys. Lett., 2014, 105, 152904 CrossRef PubMed.
- A. T. Chien, J. Sachleben, J. H. Kim, J. S. Speck and F. F. Lange, J. Mater. Res., 1999, 14, 3303–3311 CrossRef CAS.
- X.-K. Wei, Y. Su, Y. Sui, Z. Zhou, Y. Yao, C. Jin and R. Yu, Appl. Phys. Lett., 2013, 102, 242910 CrossRef PubMed.
- N. H. Hong, J. Sakai and W. Prellier, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 195204 CrossRef.
- T. Morita and Y. Cho, Appl. Phys. Lett., 2006, 88, 112908 CrossRef PubMed.
- T. Morita and Y. Cho, Appl. Phys. Lett., 2004, 85(12), 2331–2333 CrossRef CAS PubMed.
- W. S. Cho and M. Yoshimura, J. Mater. Res., 1997, 12(3), 833–839 CrossRef CAS.
- D. R. Modeshia and R. I. Walton, Chem. Soc. Rev., 2010, 39, 4303–4325 RSC.
- P. Yu, J.-S. Lee, S. Okamoto, M. Rossell, M. Huijben, C.-H. Yang, Q. He, J. Zhang, S. Yang, M. J. Lee, Q. Ramasse, R. Erni, Y.-H. Chu, D. Arena, C.-C. Kao, L. Martin and R. Ramesh, Phys. Rev. Lett., 2010, 105, 027201 CrossRef CAS.
- J. X. Ma, X. F. Liu, T. Lin, G. Y. Gao, J. P. Zhang, W. B. Wu, X. G. Li and J. Shi, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 174424 CrossRef.
- S. Smadici, B. B. Nelson-Cheeseman, A. Bhattacharya and P. Abbamonte, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 174427 CrossRef.
- K. S. Takahashi, M. Kawasaki and Y. Tokura, Appl. Phys. Lett., 2001, 79, 1324 CrossRef CAS PubMed.
- B. B. Straumal, S. G. Protasova, A. A. Mazilkin, T. Tietze, E. Goering, G. Schütz, P. B. Straumal and B. Baretzky, Beilstein J. Nanotechnol., 2013, 4, 361–369 CrossRef PubMed.
- C.-L. Tsai, Y.-J. Lina, J.-H. Chena, H.-C. Chang, Y.-H. Chen, L. Horng and Y.-T. Shih, Solid State Commun., 2012, 152, 488–492 CrossRef CAS PubMed.
- D. Gao, G. Yang, J. Zhang, Z. Zhu, M. Si and D. Xue, Appl. Phys. Lett., 2011, 99, 052502 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |