pH-responsive flower-like micelles constructed via oxime linkage for anticancer drug delivery
Bing Liu†
a,
Hongying Chen†b,
Xiao Lia,
Chaonan Zhaoa,
Yakun Liua,
Lijuan Zhuc,
Hongping Dengc,
Jichen Lia,
Guolin Li*a,
Fulin Guo*a and
Xinyuan Zhuc
aDepartment of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Nangang District, Harbin 150001, People's Republic of China. E-mail: liguolin@126.com; flguo1967@126.com; Fax: +86-451-85555710; Tel: +86-451-85555710
bDepartment of Oral and Maxillofacial Surgery, College of Stomatology, The Affiliated Hospital of Stomatology, Chongqing Medical University, Chongqing 401147, People's Republic of China
cSchool of Chemistry and Chemical Engineering, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, People's Republic of China
First published on 15th September 2014
Abstract
A new type of pH-responsive flower-like micelle based on backbone-cleavable triblock copolymer polycaprolactone-oxime-poly(ethylene glycol)-oxime-polycaprolactone (PCL-OPEG-PCL) was developed for anticancer drug delivery. Firstly, PCL-OPEG-PCL was synthesized by ring-opening polymerization. The structure of PCL-OPEG-PCL was confirmed by 1H NMR and Fourier transform infrared spectroscopy (FTIR). Benefiting from the amphiphilic character and unique molecular architecture with the hydrophilic PEG and hydrophobic PCL segments, PCL-OPEG-PCL could self-assemble into flower-like micelles in aqueous solution, which has been demonstrated by transmission electron microscopy (TEM) and dynamic light scattering (DLS). The cytotoxicity of the flower-like micelles was evaluated by MTT assay against NIH/3T3 normal cells. Doxorubicin (DOX), a model anticancer drug, was encapsulated into these flower-like micelles with high efficiency. The in vitro study showed that DOX-loaded flower-like micelles possessed high stability at physiological pH of 7.4, whereas the DOX release from the flower-like micelles was significantly accelerated at mildly acidic pH of 5.0, demonstrating the pH-responsive feature of the drug carrier with oxime linkages. DOX-loaded flower-like micelles were investigated for proliferation inhibition of Hela cells in vitro, and the DOX dose required for 50% cellular growth inhibition was found to be 1.81 μg mL−1. All of these results demonstrate that flower-like micelles self-assembled from PCL-OPEG-PCL triblock copolymers can be used as effective and promising drug nanocarriers.
Introduction
With the rapid development of therapeutic delivery technologies, polymeric vehicles self-assembled from amphiphilic polymers have become an important strategy for minimizing severe side-effects (such as cardiotoxicity and hypersensitivity) of chemotherapeutics in malignant tumor therapy.1–5 There are several unique features for polymeric micelles, such as prolonged circulation time, improved water-solubility and bioavailability of hydrophobic drugs, preferential accumulation at the tumor sites by the enhanced permeability and retention (EPR) effect and reduced systemic side effects of chemotherapeutics.6,7 However, most polymeric micelles have low responsivity at their targeting sites, which leads to low bioavailability of the therapeutic payloads.8–12To realize an efficient drug delivery, functional versatility and adaptability of polymeric micelles must be demanded. It has been reported that stimuli-responsive micelles can control the drug release by exerting an appropriate stimulus, such as temperature change, pH variation, and redox reaction.13–27 In particular, pH-sensitive polymeric micelles are one of the most promising drug delivery candidates which are responding to mild acidic surroundings in tumor tissue.13–17,28–33 It is well-known that the environment of tumor tissues is more acidic (pH = 6.5) than normal tissues and blood (pH = 7.4), and the organelles such as endosome and lysosome have even lower pH values (pH = 5.0–5.5).9,13–17 Therefore, developing smart drug delivery systems based on pH-responsive polymers for tumor therapy has attracted great attention during the past few years. To date, polymeric micelles based on acid-cleavable covalent linkages such as hydrazone, acetal, and cis-acotinyl have been designed and employed in drug carries.7,33,34 For example, Du and colleagues prepared a dual pH-sensitive polymeric drug carrier from a polymer–DOX conjugate, in which DOX was conjugated through acid-sensitive hydrazone linkages.35 Ulbrich and colleagues reported a novel pH-sensitive polymeric micellar drug delivery systems based on hydrophobic drug DOX and diblock copolymer poly(ethylene oxide)-block-poly(allyl glycidyl ether) through hydrazone linkages.36 Except for the covalent method, pH-responsive drug delivery systems based on non-covalent interactions, such as π–π stacking, hydrogen bonds and physical encapsulation, have also attracted much attention due to their universality and simplicity.7,34,37 However, it is still a challenge to develop polymeric micelles with strong stability and the ability of controllable release.18–23
With good hydrophilicity and biocompatibility, PEG have been widely applied for many clinical applications.38,39 Thus, PEG-based polymeric materials are good candidates for fabrication of biomaterials and have achieved excellent results. Especially, covalently linked amphiphilic block polymers with PEG as the hydrophilic segment have been widely used for drug delivery.40–42 Considering that oxime bonds, a new acid labile linkage with strong reversibility and dynamic ability, have been used as a robust tool to tether the segments of the block copolymer and flower-like polymeric micelles from ABA triblock copolymer possess higher physical stability than conventional micelles from AB diblock and BAB triblock polymers and,43–45 we put forward to investigate the possibility with flower-like micelles constructed by oxime linkage as a pH-responsive drug delivery system.
In this work, we systemically discussed the synthesis, characterization, in vitro cytotoxicity, and cellular uptake of the flower-like micelles from the triblock copolymer PCL-OPEG-PCL with oxime linked PEG and PCL. Because of its amphiphilicity and unique molecular architecture, the obtained triblock copolymer PCL-OPEG-PCL could self-assemble into flower-like polymeric micelles in aqueous solution. Importantly, the micelles are highly stable under physiological pH value (pH = 7.4), while they can be readily degraded in a mild acid surroundings (pH = 5–6). Compared with conventional diblock copolymer micelles, flower-like polymeric micelles exhibited the following advantages: (1) due to their flower-like architecture, they exhibited higher drug loading content and efficiency, thereby providing a sufficient concentration of DOX in the tumor cells; (2) they show lower CMC, which is beneficial for the stability of micelles, and (3) owning to oxime linkages, the flower-like micelles exhibited high pH sensitivity, which could be localized in the tumor sites by EPR effect and subsequently provoked by the acidic pH to release the drug, leading to higher anticancer efficacy with reduced side effects. To our knowledge, this is the first attempt to prepare flower-like polymeric micelles based on oxime linkage for drug delivery. The in vitro study of the drug-loaded micelles indicated the great potential of flower-like polymeric micelles as a smart drug carrier for triggered intracellular drug delivery.
Experimental section
Apparatus
Nuclear magnetic resonance (NMR) spectra were recorded on Bruker AVANCEIII 400 spectrometer with dimethyl sulfoxide-d6 (DMSO-d6), and deuterated chloroform (CDCl3) as the solvents at 25 °C. The weight-average molecular weight (Mw), number-average molecular weight (Mn) and the polydispersity (PDI) of the synthesized polymers were determined by gel permeation chromatography (GPC), which was performed on Perkin-Elmer series 200 system (10 μm PL gel 300 × 7.5 mm mixed-B and mixed-C column, polystyrene calibration) equipped with a refractive index (RI) detector. The samples were estimated by GPC with N,N-dimethylformamide (DMF) as the eluent, which contained 0.01 mol L−1 lithium bromide at a flow rate of 1 mL min−1 at 70 °C. And the data were processed with Astra software (Wyatt Technology). FTIR spectra were recorded on a Paragon 1000 instrument by KBr sample holder method. DLS measurements were recorded on a Malvern Zetasizer Nano S device equipped with a 4.0 mW laser operating at λ = 633 nm. All samples of 0.5 mg mL−1 were tested with a scattering angle of 173° at 37 °C. TEM studies were performed with a JEM-2010HT instrument operated at 200 kV to observe the shape and size of the micelles. Samples were prepared by directly dropping the solution onto carbon-coated copper grids and then air-drying at room temperature overnight before measurement. The UV-vis spectra were measured on a Perkin-Elmer Lambda 20/2.0 UV-vis spectrophotometer. The calibration curve of absorbance measurements against series of concentration of DOX was measured at 500 nm. The fluorescence spectra were recorded on a QM/TM/RM fluorescence spectrophotometer (Photon Technology International, Inc.) at room temperature.Materials
N-Hydroxyphthalimide (NOP, ShaoYuan, 97%), thriphenylphosphine (TPP, Aladdin, GR), PEG (Mn 4400–4800) (PEG-4K, ALDRICH), diisopropyl azodicarboxylate (DIAD, Aladdin, 95%), 4-(2-hydroxyethoxy)benzaldehyde (TCL, >97%) hydrazine hydrate (ACROS, 100%) ε-caprolactone (ε-CL, 98%) and stannous 2-ethylhexanoate (Sn(Oct)2, 96%) was obtained from Alfa and used as received. Toluene and methanol obtained from Sinopharm was purified by conventional method. Calcium hydride (CaH2), methylene chloride, diethyl ether, anhydrous magnesium sulfate were purchased from Sinopharm and used without any further purification. 1,6-diphenyl-1,3,5-hexatriene (DPH) were used as received without further purification. Doxorubicin hydrochloride (DOX·HCl) was purchased from Beijing Huafeng United Technology Corporation and used as received. Clear polystyrene tissue culture treated 6-well, 12-well and 96-well plates were obtained from Corning Costar. Dulbecco Clear polystyrene tissue culture, fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Biological Industries Ltd. Dialysis tube (Mw cutoff: 3500 Da) was purchased from Shanghai Lvniao Technology Corp. All other chemical reagents of analytical grade were purchased from Sinopharm and used as received unless mentioned.Synthesis of O-(N-alkoxyphthalimido)-poly(ethylene glycol) (ONPEG-4K)
A typical modification procedure was as follows. PEG-4K (20 g, 10 mmol), NOP (3.58 g, 22 mmol, 2.2 equiv.), TPP (5.5 g, 21 mmol, 2.1 equiv.) was added to a flame-dried, nitrogen purged 250 mL round-bottomed flask. After an exhausting-refilling nitrogen process three times, freshly distilled methylene chloride (100 mL) was injected and stirred at room temperature to dissolve the solids. After 15 min, DIAD (4.46 g, 4.30 mL, 22 mmol, 2.2 equiv.) was added dropwise. Each addition of DIAD made the previous orange color fade. The solution became homogenous after the addition of DIAD. Then the reaction was carried out at room temperature for another 20 h with stirring. The obtained product was concentrated and precipitated into 1.5 L of cold diethyl ether and stirred vigorously for 30 min. The products was filtered and redissolved in 50 mL methylene chloride to repeat the above process. The resulting polymer was dried overnight at 40 °C under vacuum to obtain a white powder.Synthesis of O-aminooxy-poly(ethylene glycol) (OAPEG-4K)
ONPEG-4K (20 g, 8.8 mmol) was dissolved in methylene chloride (60 mL). Hydrazine hydrate (3 mL, 24 mmol) was added dropwise and stirred vigorously at room temperature overnight. The resulting white solid was removed by filtration. The filter liquor was precipitated into 500 mL of cold diethyl ether and stirred vigorously for 30 min. The obtained white product was dried overnight at 40 °C under vacuum.Synthesis of hydroxyethyl terminal with oxime linked poly(ethylene glycol) (OAPEG4K-OH)
A typical procedure was as follows: OAPEG-4K (6.09 g, 3 mmol), 4-(2-hydroxyethoxy)benzaldehyde (1.5 g, 9 mmol) were dissolved in absolute methanol (50 mL). Anhydrous magnesium sulfate (1.5 g) was added. Then the mixture was subjected to three freeze/pump cycles and reflux for 24 h under vigorous stir. The obtained mixture was filtered and concentrated to precipitate into 500 mL of diethyl ether. The resulting polymer was dried overnight at 40 °C under vacuum to obtain a white powder.Synthesis of oxime linked triblock copolymer PCL-OPEG-PCL
The polymerization was carried out as follows. OAPEG4K-OH (1.07 g, 0.25 mmol) was added to a rond-bottomed flask. After an exhausting-refilling Ar process for three times, 20 mL anhydrous toluene was injected. Under the protection of Ar, ε-CL (1.14 g, 10 mmol) was added into the flasks with syringe. Sn(oct)2 (0.05 mmol, 20 mg) dissolved in 1 mL toluene was added. Then the bulk polymerization was carried out at 120 °C in an oil bath for 24 h with stirring. The polymerization was terminated by cooling to room temperature. The crude products were precipitated into cold methanol. The resulting polymers were filtered and dried overnight at 40 °C under vacuum to obtain a white powder.Preparation of PCL-OPEG-PCL micelles and their critical micelle concentration (CMC)
Briefly, a total of 10 mg PCL-OPEG-PCL copolymer was dissolved in 2 mL DMF and stirred at room temperature for 3 h. Then the 5 mL deionized water was slowly added into the copolymer solution under stirring for another 2 h. Thereafter, the mixture was dialyzed against the deionized water using dialysis tubing (MWCO = 3.5 KDa) for 24 h. And during the process, the water was renewed every 4 h. The CMC of obtained PCL-OPEG-PCL was determined by UV-vis spectroscopy using DPH as a probe at 313 nm. The PCL-OPEG-PCL copolymer solutions with a series of concentrations (from 1 × 10−4 to 0.1 mg mL−1) were prepared, and then methanol solution of DPH was added. The DPH concentration was kept at a constant of 5.0 × 10−6 mol L−1. The 313 nm wavelength absorbance of all solutions was recorded on Perkin-Elmer Lambda 20/2.0 UV-vis spectrophotometer.Fabrication of DOX-loaded PCL-OPEG-PCL micelles
DOX-loaded PCL-OPEG-PCL micelles were prepared via the encapsulation of hydrophobic DOX into the amphiphilic PCL-OPEG-PCL copolymer. Typically, 30 mg PCL-OPEG-PCL was dissolved in 3 mL DMSO at room temperature, according to the theoretical drug loading content (10 wt%), 3 mg DOX·HCl treated with an equal molar amount of triethylamine (TEA) was added to the polymeric solution and stirred for 3 h. Then the mixture was added slowly to 7 mL of deionized water and stirred for another 2 h. Subsequently, the solution was dialyzed against deionized water for 24 h (MWCO = 3.5 KDa), and the deionized water was renewed every 4 h. The DOX-loaded nanoparticle solution was lyophilized and then dissolved in DMSO. The DOX concentration was determined by the UV-vis measurements at absorbance wavelength of 500 nm.Drug loading content (DLC) and drug loading efficiency (DLE) were calculated according to the following formula:
| DLC (wt%) = (weight of loaded drug/weight of polymer) × 100% |
| DLE (%) = (weight of loaded drug/weight of drug in feed) × 100% |
In vitro drug release assay
The drug release study was carried out in glass bottles at 37 °C in phosphate buffer (pH = 7.4) and acetate buffer (pH = 5.0) solutions. Firstly, 2 mL DOX-loaded micelles were placed in a dialysis tube (MWCO = 3.5 KDa). Then, the dialysis bag was quickly immersed in 50 mL of the release medium in a laboratory shaker keeping the stirring (100 rpm) and a constant temperature (37 °C). At the predetermined intervals, 2 mL sample was withdrawn and the equal volume of fresh medium was replenished. The amount of released DOX was analyzed with the fluorescence measurements at 480 nm of excitation spectrum. The DOX-release studies were performed in triplicate, and the results were expressed as the average data with standard deviations.Cell culture
Both HeLa cancer cells (a human cervical carcinoma cell line) and NIH/3T3 normal cells (a mouse embryonic fibroblast cell line) were cultured in DMEM supplied with 10% FBS, and antibiotics (50 units per mL penicillin and 50 units per mL streptomycin) at 37 °C in a humidified atmosphere containing 5% CO2.Cytotoxicity measurements of PCL-OPEG-PCL micelles
The relative cytotoxicity of PCL-OPEG-PCL micelles against NIH/3T3 cells was estimated by MTT viability assay. In the MTT assay, NIH/3T3 cells were seeded into 96-well plates at a seeding density of 1.0 × 104 cells per well in 200 μL of complete medium. After 24 h of incubation, the culture medium was removed and replaced with 200 μL of a medium containing different concentrations of the PCL-OPEG-PCL micelles. The cells were grown for another 48 h. Then, 20 μL of 5 mg mL−1 MTT assays stock solution in PBS was added to each well. After incubating the cells for another 4 h, the medium containing unreacted dye was removed carefully. The obtained blue formazan crystals were dissolved in 200 μL DMSO and the absorbance was measured with BioTek® SynergyH4 at a wavelength of 490 nm.Cellular uptake of DOX-loaded PCL-OPEG-PCL micelles by HeLa cancer cells
The study of intracellular drug release was performed on the confocal laser scanning microscopy (CLSM). The details were as follows: HeLa cells were seeded into 6-well plates at 1.0 × 105 cells per well in 1 mL of complete DMEM and cultured for 24 h, followed by removing the complete medium and incubating with DOX-loaded PCL-OPEG-PCL micelles (1 mL of DMEM medium) at a final DOX concentration of 7 μg mL−1. The cells were incubated at 37 °C for predetermined intervals. Subsequently, the cells were washed twice with ice cold PBS and then fixed with 4% paraformaldehyde for 30 min at room temperature, and the slides were rinsed with ice cold PBS for three times. Finally, the cells were stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 for 5 min and the slides were rinsed with PBS for three times. The slides were mounted and observed with a LSM 510 META.
342 for 5 min and the slides were rinsed with PBS for three times. The slides were mounted and observed with a LSM 510 META.
Activity analyses
The cytotoxicity of DOX-loaded PCL-OPEG-PCL micelles and free DOX against HeLa cells was evaluated in vitro by MTT assay. HeLa cells were seeded into 96-well plates with a density 8.0 × 103 cells per well in 200 μL of medium. After 24 h of incubation, the culture medium was removed and replaced with 200 μL of a medium containing serial dilutions of DOX-loaded micelles or free DOX. The cells were grown for another 48 h. Then, 20 μL of 5 mg mL−1 MTT assays stock solution in PBS was added to each well. After incubating the cells for 4 h, the medium containing unreacted MTT was removed carefully. The obtained blue formazan crystals were dissolved in 200 μL DMSO and the absorbance was measured in BioTek® SynergyH4 at a wavelength of 490 nm.Results and discussion
Synthesis and characterization of PCL-OPEG-PCL
To construct pH-responsive polymeric micelles, the PCL-OPEG-PCL triblock copolymer containing oxime linkages in the backbone was designed and synthesized in Scheme 1. And the amphiphilic copolymer PCL-OPEG-PCL was prepared through four-step reactions, and the detailed synthetic route of the copolymer is shown in Scheme 2. Firstly, the NOP was coupled to PEG to obtain ONPEG. And after the reaction between ONPEG and hydrazine hydrate, OAPEG was obtained. To accomplish the pH-responsive property, 4-(2-hydroxyethoxy) benzaldehyde was conjugated to OAPEG through the oxime linkages to obtain the pH-sensitive product OAPEG-OH with the help of MgSO4. The hydrophobic PCL segments were grafted onto the hydrophilic PEG backbone to obtain the final products PCL-OPEG-PCL using Sn(oct)2 as the catalyst. | ||
| Scheme 1 Construction and application of pH-responsive PCL-OPEG-PCLflower-like micelles for drug delivery. | ||
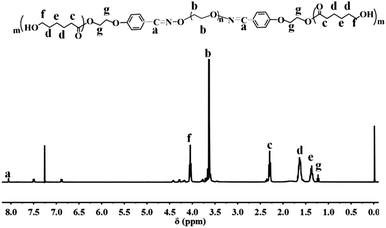
Fig. 1 shows the 1H NMR of the final copolymer product. The strong signal at 3.65 ppm is attributed to the protons of –OCH2CH2O– repeating units in PEG; while the signals at 1.20, 1.60–1.70, 2.20–2.40, and 4.20 ppm, are attributed to the protons of PCL. The appearance of proton signal at 8.00 ppm indicates the formation of oxime linkages, confirming the successful grafting of 4-(2-hydroxyethoxy)benzaldehyde onto PEG. Therefore, 1H NMR spectrum in Fig. 1 illuminates the successful synthesis of the triblock copolymer PCL-OPEG-PCL.
The PCL-OPEG-PCL was further analyzed by FTIR technique. And the representative FTIR spectrum is given in Fig. 2. In the spectrum of PCL-OPEG-PCL, a strong peak at 1727 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of esters. And the stretching of oxime bond is verified at 960 cm−1. The bands at 2945 and 2867 cm−1 correspond to asymmetric and symmetric –CH2– stretching vibrations, respectively. Besides, the strong peak at 1100 cm−1 is characteristic absorption of C–O–C. Therefore, FTIR result further supports the formation of PCL-OPEG-PCL.
O stretching vibration of esters. And the stretching of oxime bond is verified at 960 cm−1. The bands at 2945 and 2867 cm−1 correspond to asymmetric and symmetric –CH2– stretching vibrations, respectively. Besides, the strong peak at 1100 cm−1 is characteristic absorption of C–O–C. Therefore, FTIR result further supports the formation of PCL-OPEG-PCL.
Both 1H NMR and FTIR measurements confirm that the PCL segments have been successfully grafted onto the PEG backbone via oxime linkage. Additionally, GPC analysis was further employed to measure the molecular weight and PDI of PCL-OPEG-PCL. The number-average molecular weight (Mn) of PCL-OPEG-PCL determined by GPC is 4.31 × 103 with the PDI 1.332. The representative GPC profile of PCL-OPEG-PCL is shown in Fig. 3. The GPC analysis reveals a relatively unimodal and symmetric elution peak. These results indicate that the corresponding reaction is successfully achieved.
Preparation and characteristics of PCL-OPEG-PCL micelles
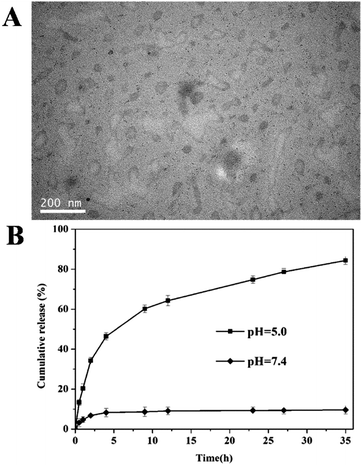
Benefiting from its amphiphilicity and unique molecular architecture, the triblock copolymer PCL-OPEG-PCL can form stable flower-like micelles in aqueous solution. The hydrophobic PCL segment serves as the inner core, while the hydrophilic PEG forms a shell to maintain a hydration barrier to provide a stable interface between the hydrophobic core and the external medium. To study the properties of the PCL-OPEG-PCL micelles, the TEM technique was employed to observe the morphologies of the PCL-OPEG-PCL micelles in aqueous solution. As shown in Fig. 4A(a), these micelles form uniform flower-like spherical structure in aqueous solution, and the average diameter is 135 nm. To further confirm the TEM observation, the DLS measurement was employed to analyze the size and distribution of the PCL-OPEG-PCL micelles. As shown in Fig. 4B(a), DLS results reveal the average hydrodynamic diameter of the PCL-OPEG-PCL micelles is about 147 nm with a PDI of 0.116, which is in accordance with the results of TEM measurement. All the data indicate that the size of flower-like micelles is suitable for drug delivery.CMC measurement of PCL-OPEG-PCL
As we know, the CMC is one of the most important parameter for micelle formation. For the CMC value of PCL-OPEG-PCL copolymer in aqueous solution, it is estimated by UV-vis technique using the DPH as a probe. As shown in Fig. 5, when the concentration of copolymer is below the CMC value, the absorbance intensity of DPH is close to zero. As soon as the hydrophobic probe is encapsulated into the PCL-OPEG-PCL micelles, the absorbance intensity of DPH strengthens exponentially with the concentration. The different two states can be reflected by two fitting lines in Fig. 5. The intersection of the horizontal line and the rapidly rising line forms the turning point, which is the CMC value of the copolymer PCL-OPEG-PCL (0.012267 mg mL−1). The detected low CMC value confirms the formation of the PCL-OPEG-PCL micelles with high stability in aqueous solution. | ||
| Fig. 5 Relationship of the absorbance intensity of DPH as a function of the triblock copolymer concentration of PCL-OPEG-PCL in aqueous solution at room temperature. | ||
The stability of PCL-OPEG-PCL micelles was also demonstrated by DLS technique. As shown in Fig. 6, no apparent change in micelle size is observed after 48 h in physiological pH of 7.4. Therefore, the high stability of PCL-OPEG-PCL flower-like micelles at physiological condition offers the possibility for the potential of therapeutic delivery.
 | ||
| Fig. 6 Size of the PCL-OPEG-PCL micelles at different time intervals in PBS (pH = 7.4) determined by DLS. Error bars represent the standard deviation (n = 3). | ||
In vitro cytotoxicity
The cytotoxicity of PCL-OPEG-PCL micelles was evaluated by MTT assay against NIH/3T3 cells. The concentration effect of PCL-OPEG-PCL micelles on the proliferation of NIH/3T3 cells is shown in Fig. 7. The MTT result indicates that the NIH/3T3 cells viability is still higher than 80% even at the highest micelle concentration (1.0 mg mL−1). Since PCL-OPEG-PCL micelles reveal the low cytotoxicity to the NIH/3T3 cells, it can be employed as a polymer vector for drug delivery.Drug loading and in vitro drug release
As one of the most potent anticancer drugs, DOX is diffusely used to treat different types of malignant tumors in clinic through interacting with DNA and inhibition of macromolecular biosynthesis.46,47 To investigate the potential of triblock copolymer PCL-OPEG-PCL micelles as drug delivery vehicle, the hydrophobic DOX was employed as the model anticancer drug to evaluate drug loading and release characteristics of PCL-OPEG-PCL micelles. The hydrophobic DOX can be encapsulated into the hydrophobic inner cores of polymeric micelles due to the hydrophobic interaction between the DOX and the PCL segments of the amphiphilic copolymer. UV-vis spectroscopy was used to measure the DLC and DLE of DOX in polymeric micelles. Based on the standard curve of the free DOX in DMSO solution at 490 nm, the DLE and DLC of the PCL-OPEG-PCL micelles were 45% and 4.5%, respectively. After the DOX loading, the size and morphology of the DOX-loaded PCL-OPEG-PCL micelles in aqueous solution were measured by TEM and DLS techniques. As shown in Fig. 4A(b), the DOX-loaded micelles form spherical micelles with the diameter of about 175 nm. And DLS result shows that the DOX-loaded micelles have an average size of 185 nm with a PDI of 0.142 in Fig. 4B(b). It should be noted that DOX-loaded PCL-OPEG-PCL micelles reveal a slight increase in the average size compared to the blank PCL OPEG-PCL micelles.The linkage of oxime bonds between hydrophobic PCL core and hydrophilic PEG shell made flower-like micelles unstable at acidic pH. To evaluate the responsive ability, the DOX-loaded micelles were treated with pH 5.0 acetate buffer (50 mM) for 12 h and the morphology of micelles was observed by TEM. As shown in Fig. 8A, exposure to acidic condition induces significant ripping or crumpling of the micelle structures, which can be attributed to the cleavage of oxime linkages, leading to the shedding of hydrophilic PEG shell from the micelles. This result suggests that the PCL-OPEG-PCL micelles possess a pH-sensitive dissolution property which can be exploited to engineer drug carriers that release their payloads under mild acidic condition in a controlled way. The release behavior of drug delivery system in the body has an important effect on its therapeutic effect. It is known that the normal pH of blood for sustaining the life is about 7.4, while the endosomes and lysosomes of cells have a more acidic microenvironment. Therefore, drug release study of DOX-loaded PCL-OPEG-PCL micelles was carried out in pH 7.4 phosphate buffer solution and pH 5.0 acetate buffer medium, respectively. As shown in Fig. 8B, the cumulative release of DOX from the DOX-loaded PCL-OPEG-PCL micelles is less than 10% after 35 h in the pH = 7.4 PBS solution. The result indicates that the PCL-OPEG-PCL micelles keep the higher stability in the physiological condition. By comparison, a noticeably increased release of DOX is observed at pH 5.0 up to 80%. Furthermore, the release rate of DOX from the PCL-OPEG-PCL nanocarriers at pH 5.0 is much faster than that at pH 7.4. The fast release of DOX from the DOX-loaded micelles in a mild acidic environment is likely due to the cleavage of oxime linkages of the triblock copolymer in acidic conditions. Meantime, the cleavage of oxime linkages further leads to damage of the micellar structure to release the encapsulated drug. It can be inferred that the DOX-loaded PCL-OPEG-PCL micelles exhibits relative high stability at neutral pH. However, when DOX-loaded PCL-OPEG-PCL micelles reach into the acid endosomes and lysosomes of the tumor cells, a large amount of drug is released to inhibit the proliferation of the cancer cells. This pH-dependent releasing behavior of the drug delivery system is very attractive for achieving the tumor-targeted programmable delivery.
Intracellular distribution assay
CLSM was exploited to investigate the intracellular distribution of the DOX-loaded micelles. DOX-loaded PCL-OPEG-PCL micelles were added to culture medium with a DOX concentration of 7 μg mL−1. HeLa cells were incubated at 37 °C for the predetermined interval 5 min, 30 min and 1 h, respectively. Subsequently, the nucleus of HeLa cells was stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342. And the blue fluorescence from Hoechst 33
342. And the blue fluorescence from Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 and red fluorescence from DOX were employed to study the intracellular localization of DOX released from the DOX-loaded PCL-OPEG-PCL micelles. As shown in Fig. 9A, the weak red fluorescence intensity is mainly located in the cytoplasm but no signal in the nucleus after incubating 5 min. After 30 min and 1 h incubation, the higher red fluorescence intensity is still located in the cytoplasm, and there is no signal in the nucleus of HeLa cells. It can be found that the concentrations of DOX-loaded PCL-OPEG-PCL micelles in cytoplasm increased gradually. It is well known that the free DOX accumulates in the nucleus by the simple passive diffusion between the extracellular and intracellular surroundings. The result indicates that the cellular uptake of DOX-loaded PCL-OPEG-PCL micelles by the cells may refer to a complicated endocytosis mechanism.
342 and red fluorescence from DOX were employed to study the intracellular localization of DOX released from the DOX-loaded PCL-OPEG-PCL micelles. As shown in Fig. 9A, the weak red fluorescence intensity is mainly located in the cytoplasm but no signal in the nucleus after incubating 5 min. After 30 min and 1 h incubation, the higher red fluorescence intensity is still located in the cytoplasm, and there is no signal in the nucleus of HeLa cells. It can be found that the concentrations of DOX-loaded PCL-OPEG-PCL micelles in cytoplasm increased gradually. It is well known that the free DOX accumulates in the nucleus by the simple passive diffusion between the extracellular and intracellular surroundings. The result indicates that the cellular uptake of DOX-loaded PCL-OPEG-PCL micelles by the cells may refer to a complicated endocytosis mechanism.
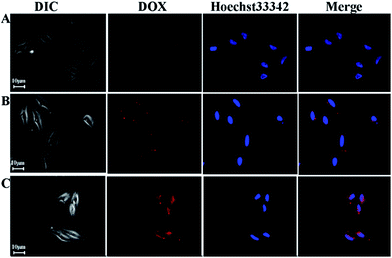 | ||
Fig. 9 CLSM images of HeLa cells incubated with DOX-loaded PCL-OPEG-PCL micelles for 5 min, 30 min and 1 h at 37 °C (DOX concentration = 7 μg mL−1). (Red: DOX, Blue: Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342). 342). | ||
In vitro cytotoxicity of the drug-loaded flower-like micelles
The drug-loaded PCL-OPEG-PCL micelles were further investigated to evaluate the potential therapeutic efficacy. The ability of DOX-loaded micelles to inhibit proliferation of HeLa cancer cells was investigated by MTT assay. To analyze the activity of DOX-loaded PCL-OPEG-PCL nanoparticles, HeLa cancer cells were cultured in the solutions of DOX-loaded micelles and free DOX at different DOX dose from 0.02 to 2.5 μg mL−1 for 48 h, respectively. As shown in Fig. 10, the dose of the loaded DOX required for 50% cellular growth inhibition (IC50) is 1.81 μg mL−1. The result indicates that DOX-loaded flower-like micelles can efficiently enter the cell and produce the desired pharmacological action. As a comparison, the IC50 of the free DOX is 1.15 μg mL−1, indicating a better cytotoxicity effect of free DOX than loaded DOX. The free DOX exhibits higher activity than that of DOX-loaded micelles, which may be due to the time-consuming DOX release from DOX-loaded micelles, confirmed by the in vitro DOX release assays. | ||
| Fig. 10 In vitro cytotoxicity DOX-loaded flower-like micelles and free DOX against HeLa cancer cells after 48 h incubation. | ||
Conclusions
A new kind of flower-like micelles with high stability and controlled release behavior was designed and prepared based on oxime-tethered triblock copolymer (PCL-OPEG-PCL) consisting of hydrophilic PEG and hydrophobic PCL. The obtained triblock copolymer PCL-OPEG-PCL was carefully characterized by 1H NMR, FTIR, and GPC techniques. Owing to its amphiphilicity and unique molecular architecture, PCL-OPEG-PCL can self-assemble into flower-like micelles which could be used as polymeric drug carriers. The self-assembled flower-like micelles possess low CMC values and desired nanosize, which endow these micelles with the improved stability and enhanced EPR effect after administration. Moreover, because of the oxime linkages, the flower-like micelles are stable in physiological conditions (pH = 7.4), while these micelles could be easily cleaved in mildly acidic conditions (pH = 5–6), resulting in rapid release of drug. The DOX-loaded PCL-OPEG-PCL micelles exhibited excellent cell internalization and high anticancer efficacy, which have been demonstrated by CLSM and MTT assay, respectively. All of these results show that these flower-like polymeric micelles with oxime linkages are favorable candidates for stimuli-responsive drug carriers.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81272466), Provincial Youth Science Fund of Heilongjiang (QC2011C037). Heilongjiang Provincial Department of Education Fund for Scientific Research Technology Project (11511168).Notes and references
- S. Aryal, M. Prabaharan, S. Pilla and S. Q. Gong, Int. J. Biol. Macromol., 2009, 44, 346–352 CrossRef CAS PubMed.
- Y. Y. Diao, H. Y. Li, Y. H. Fu, M. Han and Y. L. Hu, Int. J. Nanomed., 2011, 6, 1955–1962 CAS.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Q. Gong, Biomaterials, 2009, 30, 3009–3019 CrossRef CAS PubMed.
- W. Wang, J. X. Ding, C. S. Xiao, Z. H. Tang, D. Li, J. Chen, X. L. Zhuang and X. S. Chen, Biomacromolecules, 2011, 12, 2466–2474 CrossRef CAS PubMed.
- R. Obeid and C. Scholz, Biomacromolecules, 2011, 12, 3797–3804 CrossRef CAS PubMed.
- Q. Wang, L. J. Zhu, G. L. Li, C. L. Tu, Y. Pang, C. Y. Jin, B. S. Zhu, X. Y. Zhu and Y. Q. Liu, Macromol. Biosci., 2011, 11, 1553–1562 CAS.
- D. L. Wang, Y. Su, C. Y. Jin, B. S. Zhu, Y. Pang, L. L. Zhu, J. Y. Liu, C. L. Tu, D. Y. Yan and X. Y. Zhu, Biomacromolecules, 2011, 12, 1370–1379 CrossRef CAS PubMed.
- S. Matsumoto, R. J. Christie, N. Nishiyama, K. Miyata, A. Ishii, M. Oba, H. Koyama, Y. Yamasaki and K. Kataoka, Biomacromolecules, 2009, 10, 119–127 CrossRef CAS PubMed.
- W. Gao, J. M. Chan and O. C. Farokhzad, Mol. Pharm., 2010, 7, 1913–1920 CrossRef CAS PubMed.
- D. L. Wang, H. Y. Chen, Y. Su, F. Qiu, L. Y. Zhu, X.-Y. Huan, B. S. Zhu, D. Y. Yan, F. L. Guo and X. Y. Zhu, Polym. Chem., 2013, 4, 85–94 RSC.
- J. Chen, X. Z. Qiu, O. Y. Jun, J. M. Kong, W. Zhong and M. M. Xing, Biomacromolecules, 2011, 12, 3601–3611 CrossRef CAS PubMed.
- S. Lee, K. Saito, H. R. Lee, M. J. Lee, Y. Shibasaki, Y. Oishi and B. S. Kim, Biomacromolecules, 2012, 13, 1190–1196 CrossRef CAS PubMed.
- J. Jung, I. H. Lee, E. Lee, J. Park and S. Y. Jon, Biomacromolecules, 2007, 8, 3401–3407 CrossRef CAS PubMed.
- J. Dai, S. D. Lin, D. Cheng, S. Y. Zou and X. T. Shuai, Angew. Chem., Int. Ed., 2011, 50, 9404–9408 CrossRef CAS PubMed.
- Y. Q. Yang, W. J. Lin, B. Zhao, X. F. Wen, X. D. Guo and L. J. Zhang, Langmuir, 2012, 28, 8251–8259 CrossRef CAS PubMed.
- H. W. Sung, K. Sonaje, Z. X. Liao, L. W. Hsu and E. Y. Chuang, Acc. Chem. Res., 2012, 45, 619–629 CrossRef CAS PubMed.
- C. Giacomelli, L. L. Men and R. Borsali, Biomacromolecules, 2006, 7, 817–828 CrossRef CAS PubMed.
- G. L. Li, J. Y. Liu, Y. Pang, R.-B. Wang, L. M. Mao, D. Y. Yan, X. Y. Zhu and J. Sun, Biomacromolecules, 2011, 12, 2016–2026 CrossRef CAS PubMed.
- Y. Y. Li, X. Z. Zhang, H. Cheng, G. C. Kim, S. X. Cheng and R. X. Zhuo, Biomacromolecules, 2006, 7, 2956–2960 CrossRef CAS PubMed.
- J. Y. Koa, K. Park, Y. S. Kim, M. S. Kim, J. K. Han, K. Kim, R. W. Park, I. S. Kim, H. K. Song, D. S. Lee and I. C. Kwon, J. Controlled Release, 2007, 123, 109–115 CrossRef PubMed.
- W. Chen, F. H. Meng, R. Cheng and Z. Y. Zhong, J. Controlled Release, 2010, 142, 40–46 CrossRef CAS PubMed.
- Z. L. Sideratou, C. Kontoyianni, G. I. Drossopoulou and C. M. Paleos, Bioorg. Med. Chem. Lett., 2010, 20, 6513–6517 CrossRef CAS PubMed.
- J. W. Cui, Y. Yan, G. K. Such, K. Liang, C. J. Ochs, A. Postma and F. Caruso, Biomacromolecules, 2013, 8, 2225–2228 Search PubMed.
- X. J. Loh, J. Barrio, P. C. Toh, T. C. Lee, D. Z. Jiao, U. Rauwald, E. A. Appel and O. A. Scherman, Biomacromolecules, 2012, 13, 84–91 CrossRef CAS PubMed.
- T. Chen, M. I. Shukoor, R. W. Wang, Z. L. Zhao, Q. Yuan, S. Bamrungsap, X. L. Xiong and W. H. Tan, ACS Nano, 2011, 5, 7866–7873 CrossRef CAS PubMed.
- W. Cui, X. M. Lu, K. Cui, L. Niu, Y. We and Q. H. Lu, Langmuir, 2012, 28, 9413–9420 CrossRef CAS PubMed.
- F. X. Zhan, W. Chen, Z. J. Wang, W. T. Lu, R. Cheng, C. Deng, F. H. Meng, H. Y. Liu and Z. Y. Zhong, Biomacromolecules, 2011, 12, 3612–3620 CrossRef CAS PubMed.
- C. X. Duan, J. Gao, D. R. Zhang, L. J. Jia, Y. Liu, D. D. Zheng, G.-P. Liu, X. N. Tian, F. S. Wang and Q. Zhang, Biomacromolecules, 2011, 12, 4335–4343 CrossRef CAS PubMed.
- N. V. Rao, S. R. Mane, A. Kishore, J. D. Sarma and R. J. Shunmugam, Biomacromolecules, 2013, 1, 221–230 Search PubMed.
- Y. Z. Wang, L. Chen, Y. F. Ding and W. L. Yan, Int. J. Pharm., 2012, 422, 409–417 CrossRef CAS PubMed.
- X. Z. Yang, J. Z. Du, S. Dou, C. Q. Mao, H. Y. Long and J. Wang, ACS Nano, 2012, 6, 771–781 CrossRef CAS PubMed.
- D. Liu, H. Y. Hu, J. Zhang, X. L. Zhao, X. Tang and D. W. Chen, Chem. Pharm. Bull., 2011, 59, 63–71 CrossRef CAS PubMed.
- S. Manchun, C. R. Dass and P. Sriamornsak, Life Sci., 2012, 90, 381–387 CrossRef CAS PubMed.
- L. J. Zhu, C. L. Tu, B. S. Zhu, Y. Su, Y. Pang, D. Y. Yan, J. L. Wu and X. Y. Zhu, Polym. Chem., 2011, 2, 1761–1768 RSC.
- J. Z. Du, X. J. Du, C. Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- M. Hrubý, C. Koňák and K. Ulbrich, J. Controlled Release, 2005, 103, 137–148 CrossRef PubMed.
- Y. Jin, L. Song, Y. Su, L. J. Zhu, Y. Pan, F. Qiu, G. S. Tong, D. Y. Yan, B. S. Zhu and X. Y. Zhu, Biomacromolecules, 2011, 12, 3460–3468 CrossRef CAS PubMed.
- L. H. Piao, Z. L. Dai, M. X. Deng, X. S. Chen and X. B. Jing, Polymer, 2003, 44, 2025–2031 CrossRef CAS.
- C. B. Herold, K. Keil and D. E. Bruns, Biochem. Pharmacol., 1989, 38, 73–76 CrossRef PubMed.
- A. Mero, O. Schiavon, G. Pasut and F. M. Veronese, J. Bioact. Compat. Polym., 2009, 24, 220–234 CrossRef CAS.
- E. Locatelli and M. C. Franchini, J. Nanopart. Res., 2012, 14, 1316–1332 CrossRef.
- V. K. Garripelli, J.-K. Kim, R. Namgung, W. J. Kim, M. A. Repka and S. Jo, Acta Biomater., 2010, 6, 477–485 CrossRef CAS PubMed.
- E. S. Lee, K. T. Oh, D. Kim, Y. S. Youn and Y. H. Bae, J. Controlled Release, 2007, 123, 19–26 CrossRef CAS PubMed.
- M. A. Moretton, R. J. Glisoni, D. A. Chiappetta and A. Sosnik, Colloids Surf., B, 2010, 79, 467–479 CrossRef CAS PubMed.
- K. T. Oh, Y. T. Oh, N.-M. Oh, K. Kim, D. H. Lee and E. S. Lee, Int. J. Pharm., 2009, 375, 163–169 CrossRef CAS PubMed.
- M.-L. Adams, A. Lavasanifar and G.-S. Kwon, J. Pharm. Sci., 2003, 92, 1343–1355 CrossRef CAS PubMed.
- D.-A. Gewirtz, Biochem. Pharmacol., 1999, 57, 727–741 CrossRef CAS PubMed.
Footnote |
| † These authors are joint first authors. |
| This journal is © The Royal Society of Chemistry 2014 |