In situ functionalization of highly porous polymer microspheres with silver nanoparticles via bio-inspired chemistry†
Ho Yeon Sona,
Dong Jae Leeb,
Jun Bae Leec,
Chun Ho Parkc,
Mintae Seoa,
Jihui Jangc,
Su Ji Kimc,
Moung Seok Yoonc and
Yoon Sung Nam*ad
aDepartment of Materials Science and Engineering, Korea Advanced Institute of Science and Technology, 291 Daehak-ro, Yuseong-gu, Daejeon, 305-701, Republic of Korea
bDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, 291 Daehak-ro, Yuseong-gu, Daejeon, 305-701, Republic of Korea
cCOSMAX Research & Innovation Center, 662 Sampyong-dong, Bundang-gu, Seongnam, Gyeonggi-do 463-400, Republic of Korea
dKAIST Institute for NanoCentury (KINC CNiT), Korea Advanced Institute of Science and Technology, 291 Daehak-ro Yuseong-gu, Daejeon, 305-701, Republic of Korea. E-mail: yoonsung@kaist.ac.kr; Fax: +82-42-350-3310; Tel: +82-42-350-3311
First published on 23rd October 2014
Abstract
This study introduces a simple method to functionalize porous polymer microspheres with in situ synthesis of metal nanoparticles via mussel-inspired polydopamine chemistry. Highly open porous poly(methyl methacrylate) (PMMA) microspheres are prepared by a single oil-in-water emulsion solvent evaporation technique using Pluronic F127 as an extractable porogen. The internal pore surfaces of the prepared microspheres are coated with polydopamine via the oxidative polymerization of dopamines in an aqueous solution. The deposited polydopamine mediates the spontaneous reduction of silver nitrates into solid silver nanoparticles (Ag NPs) within the pores of the prepared microspheres, resulting in porous PMMA microspheres decorated with Ag NPs. Anti-bacterial experiments show that the Ag NP-decorated PMMA microspheres can be used as an excellent anti-bacterial platform. This study suggests that highly open porous microspheres can be used as a template to synthesize functional metal–polymer hybrid materials using the mussel-inspired polydopamine chemistry in an aqueous solution under ambient conditions.
1. Introduction
Porous polymer materials have received a great deal of attention because of their large surface area-to-volume ratio and unique architecture, both of which are very attractive for applications in catalysis, biosensors, drug delivery, and tissue engineering.1,2 Three-dimensional porous polymeric materials can be fabricated by several different techniques, including phase separation, porogen leaching, gas foaming, and colloidal templating.3–7 Recently, it was reported that hydrophobic polymer microspheres with well interconnected, macro-porous structures can be prepared by a combination of polymer phase separation and porogen leaching.8 Open pore morphology and relatively large pore sizes of the microspheres motivated us to utilize them as a porous template for the immobilization of functional inorganic nanomaterials.The incorporation of functional inorganic nanoparticles into porous polymer structures can also make unique physical and chemical advantages for various practical applications. Several methods have been suggested to produce inorganic–polymer composite materials, including the reduction of metal salts in a porous polymer matrix, the polymerization of monomers with inorganic nanoparticles, and the blending of inorganic nanoparticles with a functionalized polymer matrix.9–13 Despite many examples of inorganic–polymer composites, there are only a few examples of colloidal porous polymeric materials, in which polymer stabilizes functional inorganic nanoparticles by providing a well-defined point of attachment. In general, inorganic–polymer interfaces require a stable chemical linker that can resist chemical oxidation and hydrolysis in an aqueous milieu. Some good examples of such chemical linkages can be found in nature – mussels sticking to rocks in marine environments.
Mussel-inspired surface modification has been increasingly harnessed as a facile and robust method for the functional coatings of a broad range of substrates.14 This approach is based on the formation of polydopamine (pD) via oxidative polymerization. The pD coatings can produce a very stable polymer layer on the surface of a broad range of materials, including metal, metal oxide, polymer, ceramics, and biological cells.15–18 The catechol groups in the pD layer can react with amine and thiol groups in target molecules by Michael-type addition reactions or Schiff-base formations.19 Importantly, the catechol moiety of pD has a moderate reduction capability with a redox potential of +530 mV vs. normal hydrogen electrode (NHE) at pH 7.20 This reduction power is high enough to chemically reduce some metal ions into solid metal and metal oxide, although the reaction kinetics and the conversion efficiency may depend on experimental conditions. In particular, a metal–pD hybrid layer can be generated on various substrates through the one-pot synthesis of dopamines and metal ions, which is driven by the oxidative polymerization of dopamines and simultaneous reduction of metal ions.21
In this work we report on colloidal metal–polymer hybrid microspheres where silver nanoparticles (Ag NPs) are deposited onto the internal surfaces of porous polymer microspheres via mussel-inspired chemistry, as illustrated in Fig. 1. The pore surface of the prepared microspheres was coated with pD and exposed to a solution of Ag ions for the spontaneous reduction of Ag ions into metallic Ag NPs. The structural properties, elemental analyses, and anti-bacterial activities of the prepared Ag–pD–polymer hybrid microspheres were evaluated.
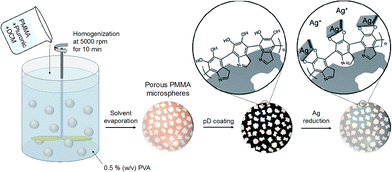 | ||
| Fig. 1 Schematic illustration of the preparation procedures of pD-coated porous PMMA microspheres and Ag–pD–PMMA hybrid microspheres. | ||
2. Experimental section
2.1 Materials
PMMA having a nominal molecular weight (Mw) of about 50 kDa was obtained from Kimex Co., Ltd (Suwon, Republic of Korea). Poly(vinyl alcohol) (PVA, 88% hydrolyzed, Mw 146–186 kDa), dopamine hydrochloride, silver nitrate (AgNO3), and Pluronic F127 were purchased from Sigma Aldrich (St. Louis, MO, USA). Dichloromethane (DCM) was obtained from Junsei Chemical (Tokyo, Japan). Milli-Q water was used as deionized water in all experiments.2.2 Preparation of porous PMMA microspheres
Highly open porous poly(methyl methacrylate) (PMMA) microspheres were prepared by an oil-in-water (O/W) emulsification technique using Pluronic F127, a tri-block copolymer comprised of poly(ethylene glycol)–poly(propylene glycol)–poly(ethylene glycol), as a water extractable porogen. This method was modified from a fabrication of a porous matrix of poly(D,L-lactide-co-glycolide) (PLGA) in a mixture of dioxane and water,3,4 and later employed to prepare porous PLGA microspheres.8 A mixture (500 mg) of PMMA and Pluronic F127 was dissolved in DCM (5 mL) in a beaker. The weight ratio of PMMA to Pluronic F127 was ranged from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 4
3 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for the preparation of porous PMMA microspheres. To prepare non-porous PMMA microspheres, only PMMA (500 mg) was dissolved in DCM (5 mL) without the addition of Pluronic F127. The PMMA–Pluronic solution was added to a 0.5% PVA solution (600 mL), followed by homogenization at 5000 rpm (PowerGen 700, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 10 min at an ambient temperature. The O/W emulsion was stirred for 24 h to completely remove DCM by evaporation. The solidified microspheres were collected by centrifugation at 1000 × g for 20 min, rinsed three times with an excess amount of deionized water, and then lyophilized.
6 for the preparation of porous PMMA microspheres. To prepare non-porous PMMA microspheres, only PMMA (500 mg) was dissolved in DCM (5 mL) without the addition of Pluronic F127. The PMMA–Pluronic solution was added to a 0.5% PVA solution (600 mL), followed by homogenization at 5000 rpm (PowerGen 700, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 10 min at an ambient temperature. The O/W emulsion was stirred for 24 h to completely remove DCM by evaporation. The solidified microspheres were collected by centrifugation at 1000 × g for 20 min, rinsed three times with an excess amount of deionized water, and then lyophilized.
2.3 Polydopamine coatings of porous PMMA microspheres
One hundred milligrams of the dried porous PMMA microspheres were dispersed in 10 mL of ethanol and incubated with mild shaking for 1 h at room temperature. The excess ethanol was removed by centrifugation at 900 × g for 10 min. The microspheres were immersed in 10 mL of a dopamine solution (2 mg mL−1) dissolved in deionized water with 0.2 mL of 0.1 M NaOH at 37 °C using a shaking incubator at 200 rpm for 1, 3, and 6 h. The pD-coated microspheres were collected by centrifugation at 900 × g for 10 min and rinsed three times with an excess amount of deionized water.2.4 Silver deposition within porous PMMA microspheres
The 1 h pD-coated porous PMMA microspheres were immersed in an aqueous solution of 1 mM AgNO3 at room temperature for 3 to 12 h with mild shaking. The sample was centrifuged at 900 × g for 10 min, rinsed three times with an excess amount of deionized water, and then lyophilized.2.5 Anti-microbial effect of Ag–pD–PMMA hybrid microspheres
The anti-bacterial activities of the prepared Ag–pD–PMMA hybrid microspheres were evaluated using Gram-negative E. coli (BL21). One hundred milligrams of the microspheres were homogeneously suspended to a 5 mL LB medium containing about 300 cells and incubated with mild shaking at 250 rpm at 37 °C in a shaking incubator. After incubation for 11 h, the LB medium containing E. coli was diluted and inoculated on solid LB-agar plates, incubated at 37 °C. After incubation for 11 h, the number of colony was counted to determine the anti-bacterial effects of the microspheres.2.6 Characterization
The morphology of the PMMA microspheres was examined using scanning electron microscopy (SEM, Hitachi S-4800) at an acceleration voltage of 10 kV after about 3 nm-thick platinum coatings. The elemental composition of the surface of PMMA microspheres was analyzed using X-ray photoelectron spectroscopy (XPS, Thermo Scientific Sigma Probe, Thermo Fisher Scientific, Inc.). The amounts of Ag NPs within the microspheres were quantitatively analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Agilent ICP-MS 7700S). The surface areas of the porous microspheres were determined by BET analysis using an AUTOSORB-1 analyzer (Quantachrome Corporation, Boynton Beach, FL, USA).3. Results and discussion
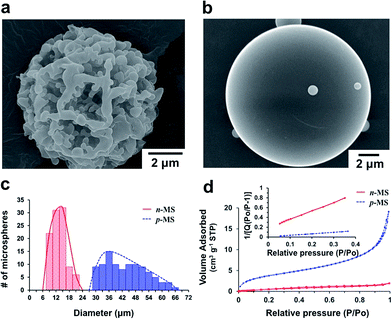
Highly open porous PMMA microspheres were prepared by a single O/W emulsification and solvent evaporation technique using Pluronic F127 as a water extractable porogen. The amphiphilic property of Pluronic F127 allows its homogeneous mixing with PMMA in a non-polar organic solvent and phase separation from PMMA when exposed to polar environments. Porous PMMA structures can be generated when Pluronic F127 is extracted from the polymer mixture using a polar solvent (e.g., water, methanol, ethanol, etc.). We prepared porous PMMA microspheres (denoted ‘p-MS’) using a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 weight ratio of PMMA and Pluronic F127 (Fig. 2a). Non-porous PMMA microspheres (denoted ‘n-MS’) were also prepared using the same procedures except the addition of Pluronic F127 (Fig. 2b). At the initial stage of emulsification, the phase separation between PMMA and Pluronic F127 might be induced by the influx of water into polymer-rich organic droplets,8 where both of PMMA and Pluronic F127 were originally dissolved in DCM. The droplets are solidified as DCM diffuses out to the aqueous phase and is eventually eliminated by evaporation. The macro-porous structures of p-MS seem to be formed as Pluronic F127 was leached out from the solidified microspheres. It should be noted that solidification of the polymer-rich droplets must occur prior to the diffusion of Pluronic F127 to the aqueous phase. Otherwise, porous structures cannot be generated because Pluronic F127 is removed from the organic phase before solidification. This phenomenon was easily observed when the top of a reactor was covered with aluminum foil or the initial volume of DCM was increased in order to retard the solvent evaporation. Fig. S1† shows that no open porous structures were generated when the volume of DCM was increased from 5 mL (100 mg mL−1) to 8 mL (62.5 mg mL−1) with the same amounts of PMMA and Pluronic F127.
5 weight ratio of PMMA and Pluronic F127 (Fig. 2a). Non-porous PMMA microspheres (denoted ‘n-MS’) were also prepared using the same procedures except the addition of Pluronic F127 (Fig. 2b). At the initial stage of emulsification, the phase separation between PMMA and Pluronic F127 might be induced by the influx of water into polymer-rich organic droplets,8 where both of PMMA and Pluronic F127 were originally dissolved in DCM. The droplets are solidified as DCM diffuses out to the aqueous phase and is eventually eliminated by evaporation. The macro-porous structures of p-MS seem to be formed as Pluronic F127 was leached out from the solidified microspheres. It should be noted that solidification of the polymer-rich droplets must occur prior to the diffusion of Pluronic F127 to the aqueous phase. Otherwise, porous structures cannot be generated because Pluronic F127 is removed from the organic phase before solidification. This phenomenon was easily observed when the top of a reactor was covered with aluminum foil or the initial volume of DCM was increased in order to retard the solvent evaporation. Fig. S1† shows that no open porous structures were generated when the volume of DCM was increased from 5 mL (100 mg mL−1) to 8 mL (62.5 mg mL−1) with the same amounts of PMMA and Pluronic F127.
The size distribution of the prepared microspheres was determined from SEM images (Fig. 2c). The average diameters of p-MS and n-MS were 43.5 ± 9.3 μm and 13.4 ± 3.4 μm, respectively. The average diameter of p-MS was much larger than that of n-MS although they were prepared under the same experimental conditions except the addition of Pluronic F127 to the organic phase. This result suggest that the infiltration of water molecules into the solidified polymer matrix could produce porous structures within the microspheres, and the generation of internal pores contributed to the increase in the overall size of the microspheres.8 The surface areas of the prepared PMMA microspheres were 13.91 m2 g−1 and 2.29 m2 g−1 for p-MS and n-MS, respectively, as determined by BET analysis (Fig. 2d), indicating that p-MS with macro-pores has much increased surface area because of its highly open porous structures. The porosity of the microspheres increased with increasing the fraction of Pluronic F127. However, if Pluronic F127 was used more than 50% of the organic phase, the resulting microspheres were so fragile that the porous structures could not be maintained. For example, non-spherical, collapsed debris were formed when a weight ratio of Pluronic F127 to PMMA was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Therefore, for the following experiments, the 5
4. Therefore, for the following experiments, the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 weight ratio of PMMA to Pluronic F127 at a concentration of 100 mg mL−1 was used.
5 weight ratio of PMMA to Pluronic F127 at a concentration of 100 mg mL−1 was used.
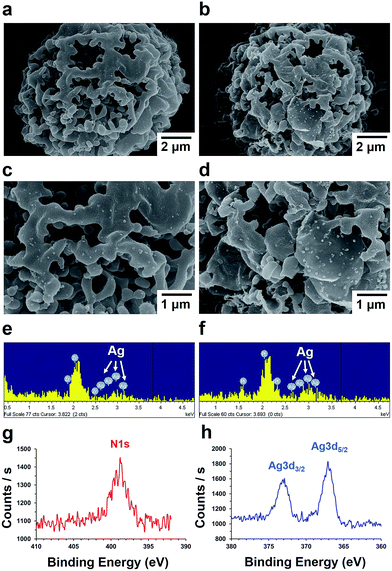
The surface of p-MS was modified using pD chemistry to introduce the strong adhesion and reducing capability to generate metal nanostructures on both of the internal and external surfaces of porous polymer microspheres. The pD layer was coated on p-MS by the oxidative polymerization of dopamines at an alkaline pH (Fig. 3a). The color of p-MS was changed from white to dark brown or black due to the catechol oxidation (Fig. 3b), and the surface of p-MS became rough due to the generation of pD aggregates on the microspheres (Fig. 3a). In alkaline condition, the catechol moiety in dopamine is oxidized to catecholquinones, subsequently rearranging to indole compounds by intramolecular cyclization of an amine group. The indole compounds are polymerized to pD covalently by further oxidation similar to the formation of melanin and/or non-covalently by the hydrogen bondings and π–π stackings.22 The catechol moiety in pD can further react with metal ions that are reduced to solid metal nanoparticles via the reducing power of the catechol moiety caused by its oxidation. The pD coating on the surface of PMMA microspheres was analyzed by XPS using 1 h pD-coated p-MS, as shown in Fig. 3c. In the XPS spectra, the nitrogen peak appeared at 398.8 eV from the pD-coated p-MS, resulting from the nitrogen in pD polymerized from dopamines, whereas no nitrogen peak appeared from the as-prepared p-MS.
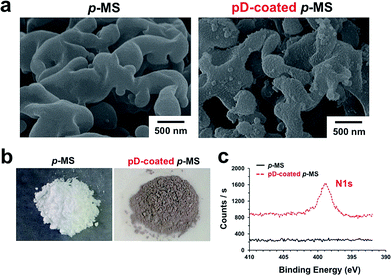 | ||
| Fig. 3 SEM images (a) and digital photographs (b) of p-MS and 1 h pD-coated p-MS. (c) XPS spectra in the nitrogen 1s binding energy region. | ||
The 1 h pD-coated microspheres were used for the preparation of Ag–pD–PMMA hybrid microspheres by immersing into an aqueous solution of 1 mM AgNO3 at ambient conditions for 3 h (denoted ‘3 h Ag–pD–PMMA’), 6 h (denoted ‘6 h Ag–pD–PMMA’) and 12 h (denoted ‘12 h Ag–pD–PMMA’). Ag NPs were spontaneously generated on the surface of the 1 h pD-coated p-MS through the oxidation of the catechol moiety on the pD layer, as shown in Fig. 4a–d. The EDX (Energy-dispersive X-ray) spectra clearly show that the surface of pD-coated p-MS was coated with Ag NPs. XPS spectra of 6 h Ag–pD–PMMA hybrid p-MS show a characteristic nitrogen peak at 398.8 eV from the pD layer and two Ag peaks at 367.1 and 372.9 eV. The amounts of Ag NPs within the microspheres (grams of Ag NPs per kilograms of the total weight of the Ag–pD–PMMA hybrid microspheres) were 1.48 g kg−1 for 3 h Ag–pD–PMMA, 4.92 g kg−1 for 6 h Ag–pD–PMMA, and 5.30 g kg−1 for 12 h Ag–pD–PMMA hybrid p-MS. The amount of Ag NPs formed on the internal surface of the microspheres increased remarkably between 3 h and 6 h of the immersing period, but afterwards no further significant reduction of Ag ions was observed probably due to the limited amounts of the catechol moiety within the microspheres.
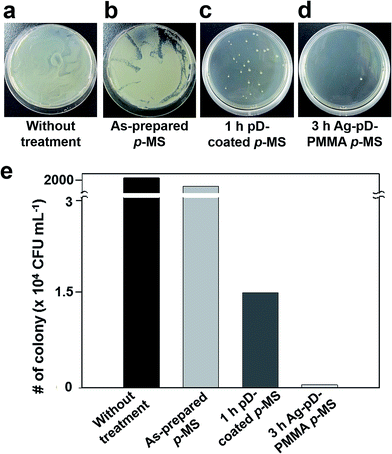
We investigated the anti-bacterial activities of the 3 h Ag–pD–PMMA hybrid microspheres against Gram-negative BL21 E. coli. The as-prepared p-MS and 1 h pD-coated p-MS were used as negative controls. The prepared hybrid microspheres were readily dispersed in water, enabling us to evaluate their anti-bacterial properties in an aqueous solution. The microspheres were dispersed in a LB medium containing E. coli at an initial cell density of 60 cells per mL. After incubation at 37 °C for 11 h, each LB medium containing E. coli and the microspheres was diluted by a factor of 102. For quantitative analysis of anti-bacterial activities of the prepared microspheres, the collected media were then inoculated on solid LB agar plates and incubated at 37 °C for 11 h to form bacterial colonies, as shown in Fig. 5a–d. The bacterial colonies were formed densely on the solid LB agar plates for negative controls incubated without any microspheres and with as-prepared p-MS, indicating that any anti-bacterial activity and cytotoxicity cannot be found from the as-prepared p-MS. The small amounts of colonies were formed for the 1 h pD-coated p-MS due to the cytotoxicity of residual dopamine monomers. Fig. 5d rarely showed the bacterial colonies, indicating the anti-bacterial activity of Ag–pD–PMMA hybrid p-MS. The number of colonies was counted to evaluate the anti-bacterial activity of each sample quantitatively. The colony densities were 2.30 × 107 CFU mL−1 for negative control incubated without any microspheres, 1.00 × 107 CFU mL−1 for the as-prepared p-MS, 1.45 × 104 CFU mL−1 for the 1 h pD-coated p-MS, and 500 CFU mL−1 for the 3 h Ag–pD–PMMA hybrid p-MS, as shown in Fig. 5e. The data clearly indicates that the Ag–pD–PMMA hybrid p-MS has a strong anti-bacterial activity by suppressing bacterial proliferation to the survival level below 0.005% in the form of suspension, which shows significantly improved anti-bacterial activity compared to Ag–polymer composite with fibrous structures and films.21,23 It has been reported that the Ag NPs have a strong bactericidal activity to E. coli. The interactions between the Ag NPs and the membrane of the E. coli. resulted in structural changes and damages to the membrane, affecting the membrane permeability that cause the degradation of the membrane as well as the cell death.24,25 In addition, the bacterial growth is significantly inhibited by the free radicals generated from Ag NPs.26 The pD-coated p-MS showed a relatively high level of anti-bacterial activity compared to p-MS without pD coatings due to residual dopamine monomers. The dopamine monomers bound non-covalently within the pD layers can be released, which are reactive to amine- and thiol-rich proteins, exhibiting low-levels of cytotoxicity.22,27 Although a small quantity of dopamine monomers is released from the pD, the concentration of pD-coated p-MS used in this study was much higher than the number of bacterial cells added into the suspension of pD-coated p-MS. We speculate that the initial growth of bacterial cells can be significantly inhibited by the relatively high concentration of dopamine monomers released from the pD-coated p-MS, exhibiting relatively high levels of anti-bacterial activity compared to the p-MS.
4. Conclusions
This study demonstrated that mussel-inspired pD chemistry provides a very useful method for functional coatings of porous colloidal structures to prepare metal–polymer hybrid materials. A simple oil-in-water emulsion solvent evaporation technique was employed to prepare highly open porous PMMA microspheres using Pluronic F127 as an extractable porogen. The prepared porous microspheres were immersed in an aqueous solution of dopamine at an alkaline pH to induce the oxidative pD polymerization on the surface of the pores. The pD-deposited surface was redox-active for the spontaneous reduction of silver precursor ions into metallic Ag NPs, producing porous Ag–pD–PMMA hybrid microspheres. The prepared Ag–pD–PMMA hybrid microspheres showed an excellent anti-bacterial activity against Gram-negative bacteria, E. coli., in a liquid test. We expect that our approach can be used as a facile method to prepare colloidal metal–polymer hybrid materials using environment-friendly processes performed in an aqueous milieu at an ambient temperature. We also expect that various functional structures comprising metal nanoparticles and polymer microstructures can be utilized for a broad range of practical applications in sensing, catalysis, electronic and biomedical applications.Acknowledgements
This study was supported by a grant of the World Class 300 Project, the Ministry of Trade, Industry & Energy (MOTIE), Republic of Korea (Grant no. 10046945) and by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111552).Notes and references
- J. A. Lee, Y. S. Nam, G. C. Rutledge and P. T. Hammond, Adv. Funct. Mater., 2010, 20, 2424–2429 CrossRef CAS.
- W. Liu, S. Thomopoulos and Y. Xia, Adv. Healthcare Mater., 2012, 1, 10–25 CrossRef CAS PubMed.
- Y. S. Nam and T. G. Park, J. Biomed. Mater. Res., 1999, 47, 8–17 CrossRef CAS.
- Y. S. Nam and T. G. Park, Biomaterials, 1999, 20, 1783–1790 CrossRef CAS.
- Y. S. Nam, J. J. Yoon and T. G. Park, J. Biomed. Mater. Res., 2000, 53, 1–7 CrossRef CAS.
- Q. Hou, D. W. Grijpma and J. Feijen, Biomaterials, 2003, 24, 1937–1947 CrossRef CAS.
- S. A. Johnson, P. J. Ollivier and T. E. Mallouk, Science, 1999, 283, 963–965 CrossRef CAS.
- H. K. Kim, H. J. Chung and T. G. Park, J. Controlled Release, 2006, 112, 167–174 CrossRef CAS PubMed.
- H. Y. Son, J. H. Ryu, H. Lee and Y. S. Nam, ACS Appl. Mater. Interfaces, 2013, 5, 6381–6390 CAS.
- J. Lee, V. C. Sundar, J. R. Heine, M. G. Bawendi and K. F. Jensen, Adv. Mater., 2000, 12, 1102–1105 CrossRef CAS.
- J.-W. Kim, J.-E. Lee, J.-H. Ryu, J.-S. Lee, S.-J. Kim, S.-H. Han, I.-S. Chang, H.-H. Kang and K.-D. Suh, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2551–2557 CrossRef CAS.
- J.-W. Kim, J.-E. Lee, S.-J. Kim, J.-S. Lee, J.-H. Ryu, J. Kim, S.-H. Han, I.-S. Chang and K.-D. Suh, Polymer, 2004, 45, 4741–4747 CrossRef CAS PubMed.
- Y.-J. Kim, J.-W. Kim, J.-E. Lee, J.-H. Ryu, J. Kim, I.-S. Chang and K.-D. Suh, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5627–5635 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- T. S. Sileika, H.-D. Kim, P. Maniak and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2011, 3, 4602–4610 CAS.
- W. Wang, Y. Jiang, S. Wen, L. Liu and L. Zhang, J. Colloid Interface Sci., 2012, 368, 241–249 CrossRef CAS PubMed.
- R. Liu, S. M. Mahurin, C. Li, R. R. Unocic, J. C. Idrobo, H. Gao, S. J. Pennycook and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 6799–6802 CrossRef CAS PubMed.
- S. H. Yang, S. M. Kang, K.-B. Lee, T. D. Chung, H. Lee and I. S. Choi, J. Am. Chem. Soc., 2011, 133, 2795–2797 CrossRef CAS PubMed.
- J. H. Ryu, Y. Lee, W. H. Kong, T. G. Kim, T. G. Park and H. Lee, Biomacromolecules, 2011, 12, 2653–2659 CrossRef CAS PubMed.
- S. Steenken and P. Neta, J. Phys. Chem., 1982, 86, 3661–3667 CrossRef CAS.
- H. Y. Son, J. H. Ryu, H. Lee and Y. S. Nam, Macromol. Mater. Eng., 2013, 298, 547–554 CrossRef CAS.
- S. Hong, Y. S. Na, S. Choi, I. T. Song, W. Y. Kim and H. Lee, Adv. Funct. Mater., 2012, 22, 4711–4717 CrossRef CAS.
- Y. Liao, Y. Wang, X. Feng, W. Wang, F. Xu and L. Zhang, Mater. Chem. Phys., 2010, 121, 534–540 CrossRef CAS PubMed.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong and M.-H. Cho, Nanomedicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- S. Hong, K. Y. Kim, H. J. Wook, S. Y. Park, K. D. Lee, D. Y. Lee and H. Lee, Nanomedicine, 2011, 6, 793–801 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of porous PMMA microspheres prepared using different volumes of DCM. See DOI: 10.1039/c4ra08685f |
| This journal is © The Royal Society of Chemistry 2014 |