K2S2O8/arenesulfinate: an unprecedented thiolating system enabling selective sulfenylation of indoles under metal-free conditions†
Abstract
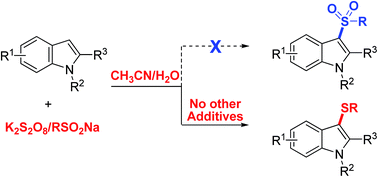
An unprecedented thiolating system K2S2O8/arenesulfinate is described for selective sulfenylation of indoles in CH3CN/H2O. This metal-free strategy enables a simple, efficient and environment-benign approach to the pharmaceutically important candidates, 3-arylthioindoles. Catalytically reactive halogen and aryl groups are well tolerated.


 Please wait while we load your content...
Please wait while we load your content...