Hydrophobic bifunctionalized hexagonal mesoporous silicas as efficient adsorbents for the removal of Orange IV†
Yongfang Zhanga,
Ling Dinga,
Wei Zhaoab,
Yunhui Zhanga,
Fei Hana,
Qingbao Fenga,
Jialiang Songa,
Meihui Lia,
Bin Dua and
Qin Wei*c
aSchool of Resources and Environmental Sciences, University of Jinan, Jinan 250022, China
bShandong Academy of Environmental Science, Jinan 250013, China
cKey Laboratory of Chemical Sensing & Analysis in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: sdjndxwq@163.com; Fax: +86-531-82765969; Tel: +86-531-82767872
First published on 24th September 2014
Abstract
A novel hydrophobic bifunctionalized hexagonal mesoporous silica (Ph-Am-HMS) material was synthesized with the simultaneous immobilization of phenyl and amino groups on its surface. The composition and structure of Ph-Am-HMS were studied by FT-IR, XRD, N2 adsorption–desorption, and sessile water droplet contact angle measurements, etc., which revealed that this novel bifunctionalized material showed hydrophobicity and good porous properties. When applied to the adsorption of the azo dye Orange IV in aqueous solutions, Ph-Am-HMS exhibited a high adsorption capacity and a rapid adsorption rate. The theoretical maximum adsorption capacity could reach 246.3 mg g−1 in 60 min at 293 K. A kinetic study indicated that the adsorption of Orange IV on Ph-Am-HMS fitted perfectly to the Freundlich adsorption model and followed pseudo-second-order kinetics. The changes in Gibbs free energy (−10.55 kJ mol−1), enthalpy (−7.06 kJ mol−1), and entropy (11.83 J mol−1 K−1) at 293 K showed that the adsorption process was spontaneous and exothermic.
Introduction
Currently, dyes and pigments are being widely used in the cosmetics, textiles, paper, plastics, pharmaceuticals and food industries. Inevitably, these synthetic dyes enter environmental water bodies through effluents from the incomplete treatment of industrial wastewater especially in developing countries, which not only affects aquatic organisms but also causes many kinds of diseases for humans such as allergic, irritant and contact dermatitis, cancers, etc.1 It is challenging work to efficiently remove these dyes, which usually have synthetic origins and complex aromatic molecular structures making them more stable and resistant to biodegradation.2 Various methods have been developed to remove these synthetic dyes from industrial wastewater over several decades, including coagulation, flocculation, adsorption, extraction, membrane filtration, resin ionic exchange, heterogeneous photocatalysis, ozone oxidation and so on.3–6 Among all the above methods for dye removal, adsorption was considered as a very convenient, efficient and inexpensive method, and has been widely used in the disposal of different dyes.7 The conventional adsorbents used in the adsorption of dye from wastewater are activated carbon, kaolin, chitosan, bentonite, titania–silica composite, etc.8–11 Due to their low adsorption efficiency, large adsorbent dosage is usually required to meet the effluent standard.In recent years, mesoporous silicas have been greatly exploited in separation, adsorption, catalysis, etc. for their fascinating properties such as high surface area, large pore volume, and big pore size.12 Better performance could be obtained from these than from the traditional microporous molecular sieves especially when big organic molecules were involved.13 Hexagonal mesoporous silica (HMS) is synthesized by a neutral S0I0 assembly pathway and possesses a much thicker framework wall, smaller domain size with short channels, and larger textual mesoporosity than its MCM-41 analogues. These properties make HMS more attractive as a new promising adsorbent.14 Furthermore, according to the application's requirements, HMS could also be flexibly modified with organic or inorganic moieties, which could increase its selective adsorption performance for different metal ions.15–17 As for the removal of organic dye, functionalized HMS showed a better performance than pure HMS, e.g., the maximum adsorption capacity for C.I. Acid Orange 10 on monoamine modified magnetic silica was 61.33 mg g−1.18 The interaction between the amino groups and the dye molecules is the key for efficient removal.
Surface hydrophilicity/hydrophobicity is one of the important properties for solid materials, and was recently found to affect the application performance when organic substrates were involved.19–21 Mesoporous silicas, due to the presence of a large amount of strong polar silanol on the surface, usually showed hydrophilicity. When used for the removal of organic molecules, due to the polarity difference, hydrophilicity might act as a negative factor for the proximity of mesoporous silica to organic molecules. In this work, a surface organic modification method was used to modify the surface hydrophilicity/hydrophobicity of HMS. Weak polar phenyl groups were introduced to modify the surface hydrophilic/hydrophobic properties by replacing the strong polar silanol to obtain a hydrophobic material. It is expected that after organic modification, the hydrophobic material might show stronger affinity to the organic molecules, and act as a more efficient adsorbent for the removal of organic dye.
Herein, a novel modified HMS was synthesized with phenyl and amino groups simultaneously immobilized on its surface. It was utilized to adsorb the acid dye Orange IV. Amino groups acted as active sites to adsorb the dye molecules, and phenyl groups were utilized to modify the surface hydrophilicity/hydrophobicity. Kinetic and thermodynamic studies were also carried out.
Experimental
Preparation of the phenyl and amino functionalized HMS
The bifunctionalized phenyl and amino HMS was synthesized at ambient temperature22,23 with the following molar composition: 0.7 TEOS; 0.2 APTES; 0.1 PTMS; 0.27 HDA; 0.27 mesitylene; 9 C2H5OH; 72 H2O. The details of the synthetic experimental procedures are given in the ESI.†Characterization
X-ray diffraction (XRD) patterns of the prepared samples were acquired at room temperature on a Rigaku D/MAX 2200 X-ray diffractometer (Tokyo, Japan) with a CuKα radiation source. The TEM images were obtained on a JEOL JEM-2100 electron microscope operated at 120 kV. FT-IR spectra (400–4000 cm−1) in KBr medium were collected using a Bruker Tensor 27 FT-IR spectrometer. Surface area measurements were performed on a Micromeritics ASAP 2020 surface area and porosity analyzer. The samples were out-gassed overnight (12 h) under nitrogen prior to adsorption measurement. Pore distributions and pore volume were calculated using the adsorption branch of the N2 isotherms based on the BJH model. The specific surface area was calculated on the basis of the BET equation. Sessile water droplet contact angles were measured with a 5 mL water droplet at ambient temperature using the contact angle measuring system JC 2000A, China. The pH values were determined using a pHS-3B pH meter (Shanghai, China).Adsorbate
Orange IV, a mono azo dye, was selected as a model dye for evaluating the potential of the prepared material to remove dye from wastewaters. It is a water-soluble anionic dye widely applied to the tinting of protein/animal fibers such as silk, wool, nylon and modified acrylic fibers. Its complete structure diagram is shown in Fig. S1 (ESI†).Batch adsorption
The adsorption behaviors of Orange IV on the prepared material were investigated using a batch method. All the experiments were repeated three times and average values were reported. The details of the adsorption tests, adsorption isotherms, and kinetic, thermodynamic, and regeneration studies are given in the ESI.†Results and discussion
Characterization of materials
In this work, bifunctionalized HMS materials with two kinds of organic groups immobilized on the surfaces of ordered mesoporous silicas were obtained through a one-step co-condensation route. Small-angle X-ray diffraction patterns of pure HMS and Ph-Am-HMS are shown in Fig. S2 (ESI†). The samples showed well-resolved patterns with an intense reflection at lower angle, which were similar to those of mesoporous materials synthesized with the same structure-directing agent, indicating that well-defined mesostructures were formed.24,25 In addition, a shift to higher angle was observed after the introduction of organic groups, indicating contraction of the lattice with phenyl and amino immobilization on the surface. This is similar to research results previously reported.22 Complementary to the XRD data, transmission electron microscopy (TEM) images revealed worm-like mesopores with channel structures throughout the sample as shown in Fig. 1.The immobilization of phenyl and amino moieties onto the surface of HMS was detected by FT-IR (Fig. S3, ESI†). From the FT-IR spectrum of pure HMS, the characteristic absorption bands near 3520, 1082, 957, and 800 cm−1 were assigned to stretching vibrations of H-bonded silanol groups ν(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH) along with physisorbed water ν(–OH), siloxane backbones ν(
Si–OH) along with physisorbed water ν(–OH), siloxane backbones ν(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Si
Si–O–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), free silanol groups ν(
), free silanol groups ν(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH), and silicon–oxygen tetrahedra ν(SiO4), respectively. The spectrum also displayed a strong band at 470 cm−1 which was assigned to ν(
Si–OH), and silicon–oxygen tetrahedra ν(SiO4), respectively. The spectrum also displayed a strong band at 470 cm−1 which was assigned to ν(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Si
Si–O–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ) deformation.18,26 The spectrum of Ph-Am-HMS displayed mainly the same characteristic bands of pure HMS as well as the strong absorption band at 3452 cm−1 assigned to N–H stretching vibrations and the abrupt absorption band at 1384 cm−1 assigned to C–N stretching vibrations. Characteristic absorption bands at 750 and 700 cm−1 might be associated with N–H bending vibrations and monosubstituted benzene out-of-plane bending vibrations, respectively. The above results gave good evidence for the successful introduction of phenyl and amino groups into the bifunctionalized HMS materials. In addition, the absorption band at about 953 cm−1 was ascribed to Si–O stretching in the Si–OH group for mesoporous siliceous material.27 Compared with the intensity of this band in the spectrum of HMS, it decreased in the spectrum of Ph-Am-HMS, which indicated that the amount of Si–OH decreased after the organic groups were introduced into the material to produce Ph-Am-HMS. Because of the polarity difference between Si–OH and the organic groups, the surface hydrophilicity/hydrophobicity might change for Ph-Am-HMS. In fact, HMS showed strong hydrophilicity with a very low sessile water droplet contact angle (15.5°, inset image in Fig. 1), while Ph-Am-HMS showed hydrophobicity with a large sessile water droplet contact angle (105.0°, inset image in Fig. 1) indicating that Ph-Am-HMS is a hydrophobic material.
) deformation.18,26 The spectrum of Ph-Am-HMS displayed mainly the same characteristic bands of pure HMS as well as the strong absorption band at 3452 cm−1 assigned to N–H stretching vibrations and the abrupt absorption band at 1384 cm−1 assigned to C–N stretching vibrations. Characteristic absorption bands at 750 and 700 cm−1 might be associated with N–H bending vibrations and monosubstituted benzene out-of-plane bending vibrations, respectively. The above results gave good evidence for the successful introduction of phenyl and amino groups into the bifunctionalized HMS materials. In addition, the absorption band at about 953 cm−1 was ascribed to Si–O stretching in the Si–OH group for mesoporous siliceous material.27 Compared with the intensity of this band in the spectrum of HMS, it decreased in the spectrum of Ph-Am-HMS, which indicated that the amount of Si–OH decreased after the organic groups were introduced into the material to produce Ph-Am-HMS. Because of the polarity difference between Si–OH and the organic groups, the surface hydrophilicity/hydrophobicity might change for Ph-Am-HMS. In fact, HMS showed strong hydrophilicity with a very low sessile water droplet contact angle (15.5°, inset image in Fig. 1), while Ph-Am-HMS showed hydrophobicity with a large sessile water droplet contact angle (105.0°, inset image in Fig. 1) indicating that Ph-Am-HMS is a hydrophobic material.
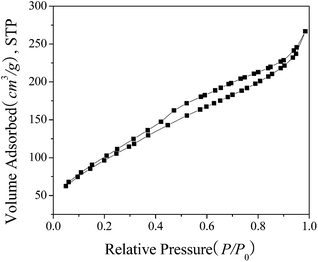
The N2 adsorption–desorption isotherm of the prepared Ph-Am-HMS material in Fig. 2 revealed a type IV isotherm with hysteresis loops according to the IUPAC classification of sorption isotherms.28 This isotherm was typical for mesoporous materials and the hysteresis loop was associated with the occurrence of pore condensation.29 The detailed structural parameters of bifunctionalized Ph-Am-HMS are listed in Table S1 (ESI†). The specific surface area of the material reached 371 m2 g−1, and the pore volume was 0.293 mL g−1. The pore size distribution calculated according to the BJH method showed that the pore diameters were in the mesoporous range with an average pore diameter equal to 3.6 nm. Compared with the data for HMS, Ph-Am-HMS showed a lower surface area, pore volume, and pore size, which should be caused by the introduction of organic groups. The introduction of organic groups usually destroys the mesoporosity during the synthesis of organic group-containing mesoporous silica.22
Comparison of different adsorbents
Performances of the hydrophobic bifunctionalized Ph-Am-HMS and hydrophilic pure siliceous HMS were studied for the adsorption of Orange IV from aqueous solutions with a contact time of 4 h. As comparisons, monofunctionalized HMS (Ph-HMS and Am-HMS) containing either phenyl or amino groups respectively were used as reference samples. The removal efficiencies of dye by different adsorbents are shown in Fig. S4 (ESI†). The results indicated that Ph-Am-HMS has the outstanding capture capacity for the dye Orange IV. It is believed that the electrostatic attraction or repulsion between the adsorbent surface and adsorbate play very important roles in adsorption processes.30 In the present work, amines, like ammonia, are weak bases in organic chemistry, and can be easily protonated at low pH (pH < 4) to produce positive charged moieties. These positive charged protonated amino groups on the silica surface were employed as efficient adsorption sites to attract the negatively charged sulfonate groups of the dye.31 In addition, introduction of hydrophobic phenyl groups into the mesopores modified the pore surface to increase its affinity to the synthetic dyes. Without organic modification, however, the surface showed hydrophilic properties due to the existence of strong polar Si–OH on the surface. Phenyl groups have more similar organic structures to the synthetic dyes molecules than do the Si–OH. According to the theory of similarity and intermiscibility, this kind of hydrophobic surface might decrease the repulsion produced by the polarity difference and then promote adsorption. Similar results were obtained in the adsorption of organic molecule when hydrophobic materials were used as adsorbents.32–35 As shown in Table 1, the comparison of saturation adsorption capacities of several organic molecules indicated that Ph-Am-HMS was an efficient hydrophobic adsorbent, especially for the removal of dye pollutants from aqueous solutions.| Adsorbent | Organic molecule | Saturation adsorption (mg g−1) | Reference |
|---|---|---|---|
| a The data in and out of the brackets are the original results cited from the references and the results after conversion to the units used in this study, respectively. | |||
| Ph-Am-HMS | Orange IV | 246.3 | This study |
| IBHS | 4-Nonylphenol | 268.8 (1.22 mmol g−1)a | 33 |
| Ph-MSN-0.75 | Methyl Orange | 10.0 (30.6 μmol g−1)a | 34 |
| Silica-sepiolite | Methyl blue | 50.2 | 35 |
In the present work, besides the surface hydrophobicity the presence of the second kind of organic group (amino group) is another guarantee of high efficiency for the adsorption of organic dye. Synergistic effects of amino and phenyl groups enable Ph-Am-HMS to be such an efficient adsorbent. In the following studies, the investigations are focused on the adsorption behavior of Orange IV by Ph-Am-HMS.
Effect of pH
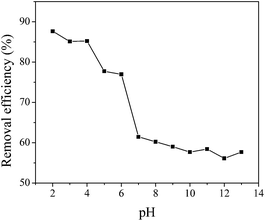
The pH value is a noteworthy parameter as it has a major effect on the protonation and deprotonation of the adsorbent and adsorbate functional groups. In this work, maximum adsorption was observed at pH 2 and the adsorption capacity remained high in the pH range 2–6 (Fig. 3). A significant decrease in the adsorption capacity occurred as the pH of the medium increased subsequently, which may be attributed to the deprotonation of amino groups along with the formation of other negatively charged silanolate groups that repel the sulfonate groups of the dye.18Adsorption kinetics
Kinetics of the Orange IV adsorption onto Ph-Am-HMS was studied for different concentrations (10, 20, 30 mg L−1) at pH 3 and 298 K. At all the concentrations, the equilibrium can be attained in about 60 min. This time is much shorter than that for other adsorbents, e.g. activated carbon, whose contaminant sorption equilibrium usually occurs after a few hours equilibration due to its significant microporosity.36,37 The rapid adsorption rate may mainly be attributed to the strong affinity of protonated amino groups toward the sulfonate groups of the dyes providing the driving force for the rapid adsorption of acid dyes. Kinetic modeling not only allows estimation of adsorption rates but also leads to suitable rate expressions characteristic of possible reaction mechanisms. In this respect, two kinetic models including the pseudo-first-order equation and pseudo-second-order equation38 were tested (the details of eqn (2) and (3) are given in the ESI†). The two kinetics of Orange IV adsorption on Ph-Am-HMS are presented in Fig. 4. The kinetic data of k, qe, and correlation coefficient (R2) calculated from eqn (2) and (3), are listed in Table S2 (ESI†). It can be seen that the kinetics of the dye adsorption on Ph-Am-HMS follows the pseudo-second-order kinetic model with correlation coefficients greater than 0.998 for all the systems in this study. | ||
| Fig. 4 Pseudo-first-order kinetics (A) and pseudo-second-order kinetics (B) of Orange IV adsorption on Ph-Am-HMS. | ||
Adsorption isotherms
It is known that the establishment of an appropriate adsorption isotherm must be taken into consideration in order to describe how adsorbates interact with adsorbents and to optimize the design of a sorption system to remove the dyes as well as for practical operation. Thus, the correlation of equilibrium data by either theoretical or empirical models is essential to practical application. In this work, the adsorption isotherms were studied using three isotherm models, Henry, Langmuir and Freundlich. The above three equations are presented in the ESI (eqn (4)–(6)).†As shown in Fig. 5, the adsorption capacity decreases noticeably as the temperature increases, which implies that Orange IV adsorbs physically on the surface of the silica material. The adsorption behavior of the dye onto Ph-Am-HMS can be well described using the Freundlich equation, and the isotherm is linear over the whole concentration range. The correlation coefficient R2, which is a measure of the goodness-of-fit, also confirms the good representation of the experimental data by the Freundlich model. The theoretical maximum adsorption capacity at 293 K is 246.3 mg g−1 on the basis of the Langmuir analysis as shown in Table S3 (ESI†).
 | ||
| Fig. 5 Henry (A), Langmuir (B) and Freundlich (C) isotherm plots for adsorption of Orange IV on Ph-Am-HMS. | ||
Adsorption thermodynamic
The thermodynamic parameters reflect the feasibility and spontaneous nature of the sorption process. The enthalpy change (ΔH°), entropy change (ΔS°) and the Gibbs free energy change (ΔG°) can be estimated using the changes in equilibrium constants with temperature (eqn (7) and (8), shown in the ESI†). The calculated thermodynamic parameters are listed in Table S4 (ESI†). In this study the value of ΔG° was determined as −10.55 kJ mol−1 at 293 K, and the value increased with an increase of temperature. The negative value of ΔG° indicates that the adsorption reactions are spontaneous. ΔH° and ΔS° values were obtained as −7.06 kJ mol−1 and 11.83 J (mol−1 K−1), respectively. The negative value of ΔH° shows that the adsorption is exothermic in nature while the positive ΔS° value reflects the increasing randomness at the solid/solution interface during the sorption of dye on the adsorbent.Adsorbent regeneration
The reusability efficiency of the adsorbent Ph-Am-HMS after two cycles remained above 99%, which indicated that it could be reused for multi-operational cycles. This is expected to reduce the cost of the sorption process in potential applications. Results related to the reuse efficiency are presented in Fig. S5 (ESI†).Conclusions
Hydrophobic bifunctionalized hexagonal mesoporous silica material containing phenyl and amino groups was synthesized through a one-step co-condensation route and employed as an adsorbent for the removal of Orange IV from aqueous solution. High efficiency was obtained with this kind of hydrophobic adsorbent due to the synergistic effect of amino and phenyl groups. The adsorption process could be well fitted by the pseudo-second order kinetic model. The equilibrium data could be well interpreted by the Freundlich isotherm. For its good porous property and specific surface hydrophobicity, this kind of novel material is expected to show good performance in the adsorption of other kinds of organic pollutant and studies are now underway.Acknowledgements
Financial support from the Doctor Foundation of Shandong Province (no. BS2010NJ002), the Natural Science Foundation of Shandong Province (no. ZR2010ZR063), and the Natural Science Foundation of China (no. 21075052, no. 21175057) is gratefully acknowledged.References
- H. Zollinger, Color Chemistry: synthesis, properties of organic dyes and pigments, VCH Publishers, New York, 1987, pp. 92–102 Search PubMed.
- S. Seshadri, P. L. Bishop and A. M. Agha, Waste Manage., 1994, 14, 127 CrossRef CAS.
- G. Ciardelli, L. Corsi and M. Marucci, Resour., Conserv. Recycl., 2000, 31, 189 CrossRef.
- P. K. Malik and S. K. Saha, Sep. Purif. Technol., 2003, 31, 241 CrossRef CAS.
- H. Kusic, D. Leszczynska, N. Koprivanac and I. Peternel, J. Environ. Sci., 2011, 23, 1479 CrossRef CAS.
- F. Deniz and S. D. Saygideger, Bioresour. Technol., 2010, 101, 5137 CrossRef CAS PubMed.
- A. Kamari, W. S. Wan Ngah, M. Y. Chong and M. L. Cheah, Desalination, 2009, 249, 1180 CrossRef CAS.
- G. M. Walker and L. R. Weatherley, Water Res., 1997, 31, 2093 CrossRef CAS.
- B. K. Nandi, A. Goswami and M. K. Purkait, Appl. Clay Sci., 2009, 42, 583 CrossRef CAS.
- P. Baskaralingam, M. Pulikesi, D. Elango, V. Ramamurthi and S. Sivanesan, J. Hazard. Mater., 2006, 128, 138 CrossRef CAS PubMed.
- G. Crini, Bioresour. Technol., 2006, 97, 1061 CrossRef CAS PubMed.
- S. Ray, M. Takafuji and H. Ihara, RSC Adv., 2013, 3, 23664 RSC.
- P. T. Tanev, M. Chibwe and T. J. Pinnavaia, Nature, 1994, 368, 321 CrossRef CAS PubMed.
- A. R. Cestari, E. F. S. Vieira and E. S. Silva, J. Colloid Interface Sci., 2006, 297, 22 CrossRef CAS PubMed.
- A. M. Liu, K. Hidajat, S. Kawi and D. Y. Zhao, Chem. Commun., 2000, 13, 1145 RSC.
- W. J. Hunks and G. A. Ozin, Chem. Commun., 2004, 21, 2426 RSC.
- O. Olkhovyk and M. Jaroniec, J. Am. Chem. Soc., 2005, 127, 60 CrossRef CAS PubMed.
- A. A. Atia, A. M. Donia and W. A. Al-Amrani, Chem. Eng. J., 2009, 150, 55 CrossRef CAS.
- M. Wang, F. Wang, J. Ma, C. Chen, S. Shi and J. Xu, Chem. Commun., 2013, 49, 6623 RSC.
- Z. Li, J. L. Nyalosaso, A. A. Hwang, D. P. Ferris, S. Yang, G. Derrien, C. Charnay, J.-O. Durand and J. I. Zink, J. Phys. Chem. C, 2011, 115, 19496 CAS.
- J. L. Hu, L. B. Luo, X. Z. Yang, R. S. Yao, H. B. Zhang and H. S. Qian, RSC Adv., 2013, 3, 25620 RSC.
- C. Chen, J. Xu, Q. Zhang, H. Ma, H. Miao and L. Zhou, J. Phys. Chem. C, 2009, 113, 2855 CAS.
- T. Yokoi, H. Yoshitake and T. Tatsumi, J. Mater. Chem., 2004, 14, 951 RSC.
- C. Chen, J. Xu, Q. Zhang, Y. Ma, L. Zhou and M. Wang, Chem. Commun., 2011, 47, 1336 RSC.
- D. A. Ruddy and T. D. Tilley, J. Am. Chem. Soc., 2008, 130, 11088 CrossRef CAS PubMed.
- J. A. A. Sales and C. Airoldi, J. Non-Cryst. Solids, 2003, 330, 142 CrossRef CAS.
- S. Schwarz, D. R. Corbin, A. J. Vega, R. F. Lobo, J. S. Beck, S. L. Suib, D. R. Corbin, M. E. Davis, L. E. Iton and S. I. Zones, Mater. Res. Soc. Symp. Proc., 1996, 431, 137 CrossRef CAS.
- S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of porous solids and powders: surface area, pore size, and density, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2004 Search PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- L. Abramian and H. El-Rassy, Chem. Eng. J., 2009, 150, 403 CrossRef CAS.
- K. Santhy and P. Selvapathy, Bioresour. Technol., 2006, 97, 1329 CrossRef CAS PubMed.
- H. Sun, A. Li, Z. Zhu, W. Liang, X. Zhao, P. La and W. Deng, ChemSusChem, 2013, 6, 1057 CrossRef CAS PubMed.
- S. Shi, M. Wang, C. Chen, F. Lu, X. Zheng, J. Gao and J. Xu, RSC Adv., 2013, 3, 1158 RSC.
- J. Peng, Y. Yao, X. Zhang, C. Li and Q. Yang, Chem. Commun., 2014, 50, 10830 RSC.
- M. Nieto-Suárez, G. Palmisano, M. L. Ferrer, M. C. Gutiérrez, S. Yurdakal, V. Augugliaro, M. Pagliaro and F. del Monte, J. Mater. Chem., 2009, 19, 2070 RSC.
- Y. P. Zhang and J. L. Zhou, Water Res., 2005, 39, 3991 CrossRef CAS PubMed.
- P. Wang, Q. H. Shi, Y. F. Shi, K. K. Clark, G. D. Stucky and A. A. Keller, J. Am. Chem. Soc., 2009, 131, 182 CrossRef CAS PubMed.
- Y. S. Ho and G. Mckay, Adsorpt. Sci. Technol., 2002, 20, 797 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08632e |
| This journal is © The Royal Society of Chemistry 2014 |