One-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under solvent-free conditions†
Ramin Ghorbani-Vaghei*,
Samira Noori,
Zahra Toghraei-Semiromi and
Zahra Salimi
Department of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, 65174, Hamedan, Iran. E-mail: rgvaghei@yahoo.com; Fax: +98 (81)38380709; Tel: +98 (81)38380709
First published on 24th September 2014
Abstract
A series of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones were obtained from aldehydes, phthalhydrazide and malononitrile in good to excellent yields at 80–100 °C under solvent-free conditions by proceeding through a simple, mild and efficient procedure utilizing N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] and poly(N-bromo-N-ethylbenzene-1,3-disulfonamide) [PBBS] as catalysts.
Introduction
Multi-component reactions (MCRs) are considered to be important concepts of organic chemistry. MCRs have great advantages over classic reaction strategies, such as greater efficiency, more irreversible product, and shorter reaction time. Multi-component reactions have now been well established as a powerful synthetic tool to produce Heterocyclic components.1,2 Organic reactions under solvent-free conditions are not only of interest from an environmental view point, but also offer considerable advantages in terms of yield, selectivity, and simplicity of the reaction procedures. Organic solvents, due to the high toxicity of the chemical industry, are a source of chemical contamination and the removal or replacement is of great help to the environment. In most cases, the reaction was performed under solvent-free conditions, better and faster than the conventional methods. The synthesis of new heterocyclic compounds has always been a subject of great interest due to their wide applicability. Heterocyclic compounds occur very widely in nature and are essential to life.3–5 Amongst a large variety of heterocyclic compounds, heterocycles containing phthalazine moiety are of interest because they show some pharmacological and biological activities.6–8 Phthalazine derivatives were reported to possess anticonvulsant,9 cardiotonic,10 vasorelaxant,11 cytotoxic,12 antimicrobial,13 antifungal,14 anticancer15 and anti-inflammatory activities.16 Recently, 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives have been synthesized by using p-toluenesulfonic acid (p-TSA) in ionic liquid, 1-butyl-3-methylimidazolium bromide [bmim]Br, as solvent at 100 °C,17 triethylamine (0.02 g, 20% mol) as catalyst in EtOH under ultrasonication18 and 1-butyl-3-methylimidazolium hydroxide ([Bmim]OH) under irradiation,19 CuI nanoparticles as catalyst under solvent-free conditions,20 Indium chloride(InCl3) as catalyst under solvent-free conditions,21 and Fe3O4 nanoparticles coated by (3-aminopropyl)-triethoxysilane as catalyst under solvent-free conditions.22 Due to the interesting properties of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, the development of synthetic methods which create a facile access to this heterocycle compounds is desirable.‡Results and discussion
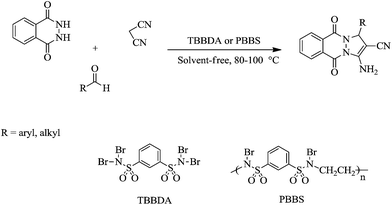
In a continuation of our interest in the application of N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] and poly(N-bromo-N-ethylbenzene-1,3-disulfonamide) [PBBS],23 in organic synthesis,23–33 we report a new and efficient method for the one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives by the condensation of various aliphatic and aromatic aldehydes with phthalhydrazide and malononitrile in the presence of TBBDA and PBBS as efficient catalysts at 80–100 °C in good to excellent yields. The route for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione is shown in Scheme 1.The advantages of TBBDA and PBBS are as follows:
1. The preparation of TBBDA and PBBS are easy.
2. TBBDA and PBBS are stable under atmospheric conditions for two months.
3. After completion of the reaction, the catalysts are recovered and can be reused several times without decreasing the yield.
Initially, we decided to explore the role of our catalyst in various conditions for the synthesis of 3-amino-5,10-dioxo-1-phenyl-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile as a model compound (Table 2, entry 1). In the ethanol system, the best results were achieved using 0.1 g of N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] (90 min, 71%, Table 1, entry 2). Therefore, we decided to test this solvent-free reaction with various ratios of TBBDA. We found that the reaction was rapid and gave excellent yield of the product while using N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] (15 min, 89%, Table 1, entry 6). In the light of this fact, subsequent studies were carried out under the following optimized conditions, that is, with 0.05 g TBBDA at 100 °C. Using a catalytic amount of aqueous 48% HBr instead of TBBDA gave lower yields (38%). This result indicates that the generation of the protic acid HBr may not be the only factor responsible for the catalytic activity of TBBDA. It is possible that the positive sulfonium moiety also has some role in facilitating. We next examined a wide variety of aldehydes containing either electron-withdrawing or electron-donating substituents successfully reacted with phthalhydrazide and malononitrile to establish the scope of catalysts (Table 1). These results encouraged us to investigate the scope and generality of this new protocol for various aliphatic and aromatic aldehydes under optimized conditions, as shown in Table 2. It is worth mentioning that there are no reports of the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones from aliphatic aldehydes. The nature and electronic properties of the aldehyde substrates affect the conversion rate and yield. Aromatic aldehydes (Table 2, entries 1–10) react faster and in higher yield than the aliphatic aldehydes (Table 2, entries 11–14).
| Entry | Solvent | Catalyst | Amount of catalyst | Time (min) | Yield (%) | Tempe (°C) |
|---|---|---|---|---|---|---|
| 1 | EtOH | TBBDA/PBBS | 0.05 g | 120 | 62/40 | Reflux |
| 2 | EtOH | TBBDA/PBBS | 0.1 g | 90 | 71/54 | Reflux |
| 3 | EtOH | TBBDA/PBBS | 0.1 g | 90 | — | rt |
| 4 | EtOH | HBr (48%) | 5 mol% | 120 | 38 | Reflux |
| 5 | Solvent-free | TBBDA/PBBS | 0.03 g | 35 | 78/61 | 100 |
| 6 | Solvent-free | TBBDA/PBBS | 0.05 g | 15 | 89/65 | 100 |
| 7 | Solvent-free | TBBDA/PBBS | 0.07 g | 15 | 89/65 | 100 |
| 8 | Solvent-free | None | — | 120 | 29 | 100 |
| Entry | Substrate | TBBDA time (min)/yield (%) [Lit.] | PBBS time (min)/yield (%) | Ref. |
|---|---|---|---|---|
| 1 | 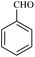 |
15/89[87] | 40/65 | 34 |
| 2 | 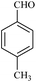 |
20/84[81] | 50/72 | 34 |
| 3 | 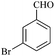 |
15/83[82] | 40/69 | 34 |
| 4 | 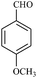 |
15/85[92] | 40/70 | 34 |
| 5 | 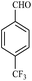 |
15/87 | 40/64 | — |
| 6 | 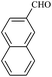 |
15/90[91] | 45/63 | 36 |
| 7 | 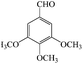 |
15/83 | 40/69 | — |
| 8 | 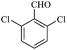 |
10/89[90] | 10/85 | 35 |
| 9 | 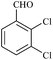 |
15/90[94] | 15/83 | 35 |
| 10 | 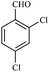 |
15/89[91] | 45/65 | 35 |
| 11 | 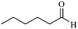 |
60/63 | 130/48 | — |
| 12 | 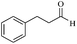 |
30/80 | 90/62 | — |
| 13 | 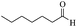 |
65/72 | 140/53 | — |
| 14 | 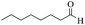 |
65/76 | 140/58 | — |
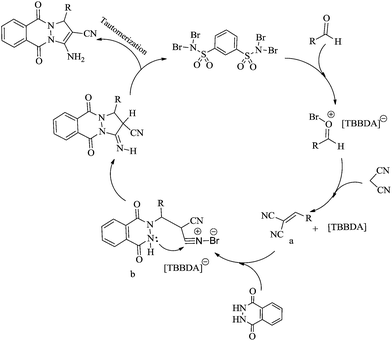
Mechanistically, it is likely that these catalysts release Br+ in situ, which can act as an electrophilic species and the mechanism shown in Scheme 2 is proposed for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives.23,32 According to this mechanism, TBBDA can be activated the carbonyl group to formation of an alkene (a) and then cation (b) as intermediates. Intramolecular cyclization lead to the 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives.
In recent years, synthesis of organic compounds in the absence of solvents has attracted much interest from chemists from the view point of reduced pollution, low cost, simplicity in process and handling.
Conclusions
We have developed a simple procedure for the synthesis of novel 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives from the reaction of various aliphatic and aromatic aldehydes with phthalhydrazide and malononitrile in the presence of TBBDA and PBBS as catalysts under solvent-free conditions. Moreover, the method has advantages in terms of product yields, recyclable catalyst, operational simplicity (easy work-up of reactions), environmental friendliness (non-corrosive catalyst), inexpensive catalyst and short reaction times.Acknowledgements
The authors are thankful to the Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), Bu-Ali Sina University, for financial support.References
- C. O. Kappe, Curr. Opin. Chem. Biol., 2002, 6, 314–320 CrossRef CAS.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef CAS.
- A. Padwa and A. G. Waterson, Curr. Org. Chem., 2000, 4, 175–203 CrossRef CAS.
- R. V. A. Orru and M. de Greef, Synthesis, 2003, 10, 1471–1499 CrossRef PubMed.
- G. Kirsch, S. Hesse and A. Comel, Curr. Org. Chem., 2004, 1, 47–63 CAS.
- F. Al-Assar, K. N. Zelenin, E. E. Lesiovskaya, I. P. Bezhan and B. A. Chahchir, Pharm. Chem. J., 2002, 36, 598–603 CrossRef CAS.
- R. P. Jain and J. C. Vederas, Bioorg. Med. Chem. Lett., 2004, 14, 3655–3658 CrossRef CAS PubMed.
- R. W. Carling, K. W. Moore, L. J. Street, D. Wild, C. Isted, P. D. Lesson, S. Thomas, D. O,cooner, R. M. Mckernan, K. Quirk, S. M. Cook, J. R. Atach, K. A. Waftord, S. A. Thompson, G. R. Dawson, P. Ferris and J. L. Castro, J. Med. Chem., 2004, 47, 1807–1822 CrossRef CAS PubMed.
- S. Grasso, G. Desarro, N. Micale, M. Zappala, G. Puia, M. Baraldi and C. Demicheli, J. Med. Chem., 2000, 43, 2851–2859 CrossRef CAS PubMed.
- Y. Nomoto, H. Obase, H. Takai, M. Teranishi, J. Nakamura and K. Kubo, Chem. Pharm. Bull., 1990, 38, 2179–2183 CrossRef CAS.
- N. Watanabe, Y. Kabasawa, Y. Takase, M. Matsukura, K. Miyazaki, H. Ishihara, K. Kodama and H. Adachi, J. Med. Chem., 1998, 41, 3367–3372 CrossRef CAS PubMed.
- S. Grasso, G. DeSarro, N. Micale, M. Zappala, G. Puia, M. Baraldi and C. Demicheli, J. Med. Chem., 2000, 43, 2851–2859 CrossRef CAS PubMed.
- N. Watanabe, Y. Kabasawa, Y. Takase, M. Matsukura, K. Miyazaki, H. Ishihara, K. Kodama and H. Adachi, J. Med. Chem., 1998, 41, 3367–3372 CrossRef CAS PubMed.
- Y. Nomoto, H. Obase, H. Takai, M. Teranishi, J. Nakamura and K. Kubo, Chem. Pharm. Bull., 1990, 38, 2179–2183 CrossRef CAS.
- A. A. Bekhit, H. T. Y. Fahmy, S. A. F. Rostom and A. M. Baraka, Eur. J. Med. Chem., 2003, 38, 27–34 CrossRef CAS.
- A. I. Eid, M. A. Kira and H. H. Fahmy, J. Pharm. Belg., 1978, 33, 303–311 CAS.
- R. Ghahremanzadeh, G. I. Shakibaei and A. Bazgir, Synlett, 2008, 8, 1129–1132 Search PubMed.
- M. R. Nabid, S. J. Tabatabaie, R. Gahremanzadeh and A. Bazgir, Ultrason. Sonochem., 2010, 17, 159–161 CrossRef CAS PubMed.
- D. S. Raghuvanshi and K. N. Singh, Tetrahedron Lett., 2011, 52, 5702–5705 CrossRef CAS PubMed.
- J. Safaei-Ghomi, H. Shahbazi-Alavi, A. Ziarati, R. Teymuri and M. R. Saberi, Chin. Chem. Lett., 2014, 25, 401–405 CrossRef CAS PubMed.
- M. Veeranarayana Reddy and Y. Tae Jeong, Tetrahedron Lett., 2013, 54, 3546–3549 CrossRef CAS PubMed.
- H. R. Shaterian and M. Mohammadnia, Res. Chem. Intermed., 2014, 40, 371–383 CrossRef CAS.
- R. Ghorbani-Vaghei and H. Jalil, Synthesis, 2005, 4, 1099–1102 CrossRef PubMed.
- R. Ghorbani-Vaghei, E. Shahbazee and H. Veisi, Mendeleev Commun., 2005, 15, 207–208 CrossRef PubMed.
- R. Ghorbani-Vaghei and E. Shahbazee, J. Braz. Chem. Soc., 2005, 16, 647–649 CrossRef CAS PubMed.
- M. A. Zolfigol, R. Ghorbani-Vaghei, S. Mallakpour, G. Chehardoli, A. Ghorbani-Choghamani and A. Hosain Yazdi, Synthesis, 2006, 10, 1631–1634 CrossRef PubMed.
- R. Ghorbani-Vaghei and S. Akbari-Dadamahaleh, Tetrahedron Lett., 2009, 50, 1055–1058 CrossRef CAS PubMed.
- R. Ghorbani-Vaghei, Tetrahedron Lett., 2003, 44, 4529–7532 Search PubMed.
- R. Ghorbani-Vaghei, H. Veisi, H. Keypour and A. Dehghani-Firouzabadi, Mol. Diversity, 2010, 14, 87–96 CrossRef CAS PubMed.
- (a) R. Ghorbani-Vaghei, M. Amiri, R. Karimi-Nami and Z. Salimi, RSC Adv., 2013, 3, 25924 RSC; (b) R. Ghorbani-Vaghei, Z. Salimi, S. M. Malaekehpoor, F. Eslami and S. Noori, RSC Adv., 2014, 4, 33582–33586 RSC.
- R. Ghorbani-Vaghei, Z. Toghraei-Semiromi and R. Karimi-Nami, J. Braz. Chem. Soc., 2011, 22, 905–909 CAS.
- R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri and M. Ghavidel, Tetrahedron, 2011, 67, 1930–1937 CrossRef CAS PubMed.
- R. Ghorbani-Vaghei, H. Shahbazee and H. Veisi, Tetrahedron Lett., 2012, 53, 2325–2327 CrossRef CAS PubMed.
- S. H. Song, J. Zhong, Y. H. He and Z. Guan, Tetrahedron Lett., 2012, 53, 7075–7077 CrossRef CAS PubMed.
- H. R. Shaterian and M. Mohammadnia, J. Mol. Liq., 2012, 173, 55–61 CrossRef CAS PubMed.
- A. Azarifar, R. Nejat-Yami and D. Azarifar, J. Iran. Chem., 2013, 10, 297–306 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08617a |
| ‡ Materials and Equipment. All commercially available chemicals were obtained from Merck and Fluka and used without further purification unless otherwise stated. 1H and 13C-NMR spectra were recorded on Bruker Avance 300 FT NMR spectrometers (undertaken at Kharazmi University, Iran), 400 FT NMR spectrometers (undertaken at University of Isfahan, Iran) and Jeol FT-90 NMR spectrometers (undertaken at Bu-Ali Sina University, Iran). Mass spectra were recorded on a 5973 Network Mass selective Detector Mass Spectrometer (undertaken at University of Tehran, Iran). Elemental analyses (CHN) were performed with a Elemental Combustion System 4010 (undertaken at University of Tehran, Iran). Typical experimental procedure for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives using TBBDA and PBBS catalysts: A mixture of malononitrile (2 mmol), phthalhydrazide (2 mmol), aldehyde (2.2 mmol), and TBBDA (0.09 mmol) or PBBS (0.1 g) was heated at 80 °C. After completion of the reaction by TLC [chamber containing iodine crystals and ethyl acetate/n hexane (3 3-Amino-5,10-dioxo-1-pentyl-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile 11: MS: m/z: 310, 239, 232, 184, 162, 155, 132, 119, 104, 76, 57, 51. Anal. calcd for C17H18N4O2: C, 65.79; H, 5.85; N, 18.05. Found: C, 65.69; H, 5.73; N, 17.47. 3-Amino-1-hexyl-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile 13: MS: m/z: 324, 239, 184, 162, 130, 119, 104, 76, 50. Anal. calcd for C18H20N4O2: C, 66.65; H, 6.21; N, 17.27. Found: C, 66.64; H, 6.17; N, 17.13. 3-Amino-1-heptyl-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile 14: MS: m/z: 338, 239, 184, 162, 130, 104, 76, 50. Anal. calcd for C19H22N4O2: C, 67.44; H, 6.55; N, 16.56. Found: C, 67.03; H, 6.24; N, 16.83. 3-Amino-5,10-dioxo-1-phenethyl-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile 12: MS: m/z: 344, 329, 239, 184, 162, 155, 130, 104, 91, 76, 50. Anal. calcd for C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.55; H, 4.38; N, 16.64. |
| This journal is © The Royal Society of Chemistry 2014 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: