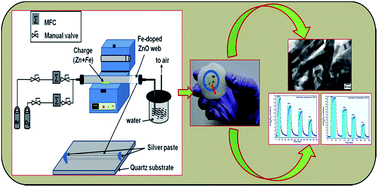
Herein, we demonstrate the growth of Fe-doped ZnO nanowires using a typical vapour phase transport process for oxygen gas sensing application. The morphologies of these nanowires have been evaluated by high-resolution transmission electron microscopy, which confirms the formation of ZnO nanowires with 20–50 nm diameter and with a d-spacing of ∼0.270 nm, corresponding to (002) planes. The energy dispersive X-ray spectroscopy result reveals the presence of Fe, Zn and O elements in 9 wt% Fe-doped ZnO nanowires. The Raman and photoluminescence spectroscopy results explore the oxygen deficient structure of these nanowires with Fe ion occupying tetrahedral sites in the ZnO lattice along with some interstitial Zn and Fe ions. Furthermore, these nanowires arrays illustrate a sensitivity of 23 and 31 with fast response/recovery times of about 38 s/46 s and 11 s/11 s at an operating temperatures of 100 °C and 140 °C, respectively. The obtained results established that these luminescent ZnO nanowires could be an excellent choice for high-performance oxygen gas sensing application.