Fabrication of self-assembling peptide nanofiber hydrogels for myocardial repair
Abstract
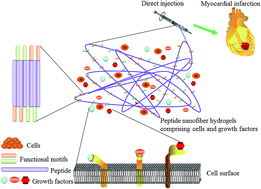
Myocardial infarction (MI) has become widely spread in clinical medicine, and results in massive loss of myocardium, ventricular remodeling and ultimately heart failure. Many strategies for repairing injured myocardium are under extensive investigation with some early encouraging effects, but cannot effectively prevent disease progression and heart failure. Cardiac tissue engineering has emerged as an increasingly important approach to repair infarcted myocardium. Self-assembling peptide nanofiber hydrogels are regarded as tailored biomaterial scaffolds with the aim to develop cardiac tissue engineering. Their fabrication is based on the principle of molecular self-assembly which allows tailoring of the biological features (e.g. modification with functional motifs and controlled release of signal molecules) of supramolecular structures for cell behaviors and tissue regeneration. This review will outline the potential of combinational treatments of peptide nanofiber hydrogels with cells, growth factors, or both together in myocardial repair.

 Please wait while we load your content...
Please wait while we load your content...