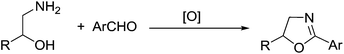Transition metal and base free synthesis of 2-aryl-2-oxazolines from aldehydes and β-amino alcohols catalysed by potassium iodide†
C. Uma Maheswari*a,
G. Sathish Kumarb and
M. Venkateshwarb
aDepartment of Chemistry, School of Chemical and Biotechnology, SASTRA University, Thanjavur-613401, India. Fax: +91-4362-264111; Tel: +91-4362-304346E-mail: uma.cchem@gmail.com
bInorganic and Physical Chemistry Division, Indian Institute of Chemical Technology, Tarnaka, Hyderabad 500607, India
First published on 21st August 2014
Abstract
Synthesis of 2-aryl-2-oxazolines from β-amino alcohols and aldehydes was achieved in good to excellent yield by employing a potassium iodide (KI)–tert-butyl hydroperoxide (TBHP) catalytic system. This protocol is very mild, metal and base free and can be performed under ambient reaction conditions. This oxidative cyclization strategy was further extended for the synthesis of optically active 2-oxazolines, which can act as very useful chiral auxiliaries and as ligands.
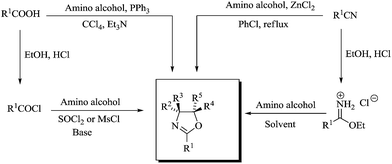
Construction of nitrogen containing five-membered heterocycles is an important synthetic strategy for organic chemists due to their wide range of applicability in syntheses of various biologically active compounds and their scope as ligands in homogeneous asymmetric catalysis. Among them, 2-oxazolines (4,5-dihydrooxazoles) exist in a variety of natural products, bio-active compounds and also as enzyme inhibitors.1 Oxazoline derivatives exhibit various pharmaceutical activities such as anti-diabetic,2 antihypertensive,3 anti-depressive,4 anticancer,5 anti-HIV-16 and antitumor7 activities to name a few. Chiral mono- and bis-oxazolines are used as chiral auxiliaries and as ligands in asymmetric synthesis.8 Achiral oxazolines finds their use as an important protecting group and as a valuable intermediate in organic synthesis.9 Numerous methods for the construction of 2-oxazolines have been reported (Scheme 1), from carboxylic acids,10 esters,11 nitriles,12 aldehydes,13 and olefins.14
Despite a certain degree of success in the above mentioned methods, they have several limitations like multi-step synthesis and significant amount of by-product formation. Moreover, some of these methods require elaborate purification process and harsh reaction conditions. Oxidative methods for the formation of heterocyclic compounds are quite attractive and several methods were already reported for the formation of benzoxazoles, benzimidazoles and imidazolines from aldehydes.15
However, synthesis of oxazolines by oxidative strategy from aldehydes was little explored and was performed using pyridinium hydrobromide perbromide (PHPB),16 N-bromosuccinimide (NBS),17 and 1,3-diiodo-5,5-dimethylhydantoin (DIH).18 The main drawbacks of these procedures are: they use stoichiometric amount of reagents to facilitate the conversion in the presence of excess base. This in turn leads to excess amount of inorganic salts as by-product. To circumvent this problem associated with stoichiometric process, they can be replaced by environmentally friendly catalytic oxidative methods which comes under the well-known term “green chemistry” as proposed by Anastas and Warner.19 In catalytic oxidation, the stoichiometric oxidants used for synthesis of fine chemicals are molecular oxygen, hydrogen peroxide, alkyl hydroperoxides especially tert-butyl hydroperoxide (TBHP), persulfate, percarbonate, hypochlorite and hypochlorates to name a few.20 In similar lines, there are few reports on TBHP mediated oxidative cyclization for the synthesis of heterocycles. For e.g. for the synthesis of benzothiazoles,21 pyrazoles,22 oxazoles,23 2-phenylquinazolines,24 in the presence of iodine or transition metals like Cu or Fe. But there are no reported literatures for the synthesis of 2-oxazolines. Thus formation of 2-aryl-2-oxazolines by oxidative cyclization starting from readily available aldehydes and β-amino alcohols prove to be an important strategy (Scheme 2).
We have successfully demonstrated the catalytic oxidative transformations viz., synthesis of 3H-quinazolin-4-ones and 4H-3,1-benzoxazin-4-ones,25 amidation of aldehydes and alcohols,26 selective oxidation of aromatic amines to nitro compounds27 using catalytic amount potassium iodide in the presence of TBHP as the external oxidant. As part of our ongoing study on iodine/iodide mediated catalytic oxidative functionalization, herein, we would like to present a mild and selective synthesis of 2-aryl-2-oxazolines using KI–TBHP catalytic system.
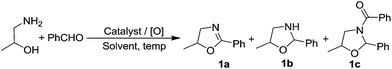
For our initial optimization studies, benzaldehyde and 1-amino-2-propanol was taken as the model substrates (Table 1). Blank reactions without oxidant and catalyst (KI or I2) proved to be futile and yielded only oxazolidine (1b) as the major product (entries 1–3). When KI in combination with TBHP in dil. AcOH was taken, the required 2-oxazoline (1a) was formed along with an amide (1c) (entry 4). Similar observation was made using water as the solvent (entry 5). However, drastic improvement in product selectivity was observed shifting from aqueous to organic solvents. Among different organic solvents screened, CH2Cl2 proved to be the best solvent in terms of conversion and selectivity (entry 8). Either the change of oxidant (from TBHP to NaOCl) or decrease in catalyst loading (from 20 mol% to 10 mol%) could not improve the product conversion or selectivity (entries 10–12). But when 10 mol% of KI was used and the reaction time was prolonged for 12 h, we could observe comparable yields as 20 mol% catalyst loading with 6 h reaction time (entry 13). Thus KI in conjunction with TBHP as the oxidant in CH2Cl2 at room temperature was taken as the optimized reaction condition for oxidative cyclization (entry 13).
| S. no. | Catalyst (mmol) | [O] | Solvent | Conversionb (%) | Selectivityb | ||
|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | |||||
| a Reaction conditions: 1-amino-2-propanol (1.2 mmol), benzaldehyde (1 mmol), KI (0.2 mmol), 70% aq. TBHP (3.0 mmol), solvent (3 mL), rt, 6 h.b Conversion and selectivity based on GC.c Reaction at 80 °C.d Simple amide (N-(2-hydroxypropyl)benzamide) was observed as the major product.e Reaction time = 12 h. | |||||||
| 1 | — | — | AcOH | 58 | — | 100 | — |
| 2 | — | TBHP | AcOH | 60 | 06 | 83 | — |
| 3 | KI | — | AcOH | 64 | — | 100 | — |
| 4 | KI | TBHP | AcOH | 95 | 45 | — | 27 |
| 5 | KI | TBHP | H2O | 93 | 42 | — | 19 |
| 6 | KI | TBHP | CH3CN | 67 | 54 | 18 | 16 |
| 7 | KI | TBHP | CH3CNc,d | 63 | 40 | 16 | — |
| 8 | KI | TBHP | THF | 48 | 05 | 85 | — |
| 9 | KI | TBHP | CH2Cl2 | 92 | 88 | — | — |
| 10 | KI | TBHP | Toluene | 75 | 55 | 06 | — |
| 11 | KI | NaOCl | CH2Cl2d | 84 | 03 | 46 | — |
| 12 | KI (0.1) | TBHP | CH2Cl2 | 64 | 55 | 33 | — |
| 13 | KI (0.1) | TBHP | CH2Cl2e | 89 | 86 | — | — |
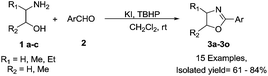
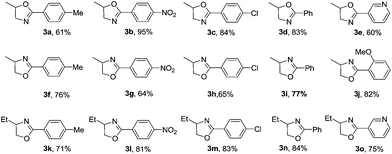
The general applicability of this reaction was evaluated with structurally diverse aldehydes and amino alcohols (Table 2). In general the yields are moderate to good with different amino alcohols and aldehydes. However, there was a slight variation in the product yields depending on the position of the substitution on the amino alcohol as well as aldehydes. For example, in case of 1-amino-2-propanol, electron withdrawing groups on aldehydes gave better yields compared to the electron donating substrates (entries 1–5). In case of 2-amino-1-propanol, yields are better with electron donating substrates (entries 6–10). No such trend was observed when 2-amino-1-butanol was taken as the amino alcohol variant. The yields are generally good with both electron withdrawing and electron donating aldehydes (entries 11–15). When we employed aliphatic aldehyde, butyraldehyde as aldehyde source with 1a for this reaction, the yield decreased considerably (<20%). Thus this methodology was best suited for the synthesis of 2-aryl-2-oxazolines.
This oxidative cyclization strategy was applied for the synthesis of optically active 2-oxazolines, which are very useful as chiral auxiliaries and as ligands (Table 3). The reaction with benzaldehyde and various chiral amino alcohols (>98% ee) provided the product with good yields and good optical purity (entries 16–20). There is no improvement in optical purity of the product, even when the reactions were carried out at lower temperature (0 °C). The slight drop in optical purity may be explained on the basis of imine–enamine tautomerism, which accounts for partial racemization.
| Entry | Amino-alcohol | Product | Yieldb (%) | Optical purityc (%) |
|---|---|---|---|---|
| a Reaction conditions: amino-alcohol (1.2 mmol), aldehyde (1 mmol), CH2Cl2 (3 mL), KI (0.1 mmol), TBHP (3.0 mmol), 10 h.b Isolated yield.c Optical purity based on optical rotation.d TBHP in decane was used. | ||||
| 1 | 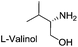 |
 |
81 | 92, 96d |
| 2 | 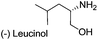 |
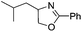 |
68 | 86 |
| 3 | 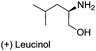 |
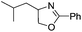 |
67 | 93 |
| 4 | 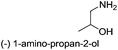 |
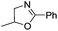 |
78 | 90 |
| 5 | 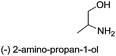 |
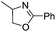 |
74 | 93 |
Since chiral oxazolines are very useful chiral auxiliaries and important class of ligands, the feasibility of the present catalytic system on multi-gram scale was examined for the synthesis of 3p [(S)-4-isopropyl-2-phenyl-4,5-dihydrooxazole]. Reaction of commercially available chiral amino alcohol, L-valinol (5 g, 48.5 mmol, 1.2 equiv.) with benzaldehyde (1 equiv.), KI (0.1 equiv.) and 3.0 equiv. of TBHP at room temperature in 40 mL of CH2Cl2 provided 3p in 80% (6.12 g) isolated yield after 12 h with good ee of 90.6%.
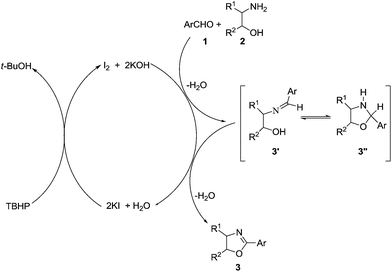
A plausible mechanism for the formation of 2-aryl-2-oxazolines was described as in Scheme 3. The initial step could be the formation of imine 3′. Iodine acts as a mild Lewis acid, thus facilitating the intra-molecular cyclization of 3′ to give oxazolidine 3′′. Oxazolidine in presence of oxidant undergoes oxidative dehydrogenation to yield the desired 2-aryl-2-oxazolines under the present reaction condition.
Conclusions
In summary, we have developed a simple and straightforward method for the synthesis of the 2-aryl-2-oxazolines by oxidative cyclization of aldehydes and an amino-alcohol using KI–TBHP. This method eliminates the use of transition metal, stoichiometric reagents and base for the formation of 2-aryl-2-oxazolines, a biologically active heterocyclic compound. Moreover, the reaction was carried out at ambient conditions and no other side products are obtained. The present methodology was also extended to chiral amino alcohol derivatives to yield chiral oxazolines, which are important as ligands and auxiliaries. In addition, the present non-transition metal-oxidant catalytic system also provides an easy scale-up and separation protocol.Acknowledgements
CUM thanks SASTRA University's in house T.R. Rajagopalan funding program. She also thanks DST, SERB, New Delhi for the award of a research grant for young scientist (no. SB/FT/CS-108/2012). She thanks Dr K. Rajender Reddy, Senior Scientist, I&PC Division, Indian Institute of Chemical Technology (IICT), Hyderabad, India for his constant motivation and suggestions.Notes and references
- J. V. Greenhill and L. Lue, in Progress in Medicinal Chemistry, ed. G. P. Ellis and D. K. Luscombe, Elsevier, New York, 1993, vol. 3 Search PubMed.
- F. Rondu, G. Lr Bihan, X. Wang, A. Lamouri, E. Touboul, G. Dive, T. Bellahsene, B. Pfeiffer, P. Renard, B. Guardiola-Lemaıtre, D. Manéchez, L. Pénicaud, A. Ktorza and J. J. Godfroid, J. Med. Chem., 1997, 40, 3793 CrossRef CAS PubMed.
- P. Bousquet and J. Feldman, Drugs, 1999, 58, 799 CrossRef CAS PubMed.
- E. S. Vizi, Med. Res. Rev., 1986, 6, 431 CrossRef CAS PubMed.
- P. Wipf and P. C. Fritch, Tetrahedron Lett., 1994, 35, 5397 CrossRef CAS.
- R. J. Boyce, G. C. Mulqueen and G. Pattenden, Tetrahedron Lett., 1994, 35, 5705 CrossRef CAS.
- P. Wipf and S. Venkatraman, Synlett, 1997, 1 CrossRef CAS PubMed.
- G. Desimoni, G. Faita and K. A. Jorgensen, Chem. Rev., 2006, 106, 3561 CrossRef CAS PubMed and references cited therein.
- T. W. Greene and P. G. M. Wuts, Protecting Groups in Organic Synthesis, John Wiley, New York, 2nd edn, 1991 Search PubMed.
- (a) P. Vastila, I. M. Pastor and H. Adolfsson, J. Org. Chem., 2005, 70, 2921 CrossRef PubMed; (b) S. Rajaram and M. S. Sigman, Org. Lett., 2002, 4, 3399 CrossRef CAS PubMed; (c) C. O. Kangani and D. E. Kelley, Tetrahedron Lett., 2005, 46, 8917 CrossRef CAS PubMed; (d) A. Cwik, Z. Hell, A. Hegedus, Z. Finta and Z. Horvath, Tetrahedron Lett., 2002, 43, 3985 CrossRef CAS.
- T. Ohshima, T. Iwasaki and K. Mashima, Chem. Commun., 2006, 2711 RSC.
- (a) I. M. Baltork, V. Mirkhani, M. Moghadam, S. Tangestaninejad, M. A. Zolfigol, M. A. Alibeik, A. R. Khosropour, H. Kargar and S. F. Hojati, Catal. Commun., 2008, 9, 894 CrossRef PubMed; (b) I. M. Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani and S. F. Hojati, Catal. Commun., 2008, 9, 1153 CrossRef PubMed.
- (a) P. Chaudhry, F. Schoenen, B. Neuenswander, G. H. Lushington and J. Aube, J. Comb. Chem., 2007, 9, 473 CrossRef CAS PubMed; (b) D. B. Garagorri, V. Bocokic and K. Kirchner, Tetrahedron Lett., 2006, 47, 8641 CrossRef PubMed.
- (a) S. Minakata, M. Nishimura, T. Takahashi, Y. Oderaotoshi and M. Komatsu, Tetrahedron Lett., 2001, 42, 9019 CrossRef CAS; (b) S. Hajra, S. Bar, D. Sinha and B. Maji, J. Org. Chem., 2008, 73, 4320 CrossRef CAS PubMed; (c) S. Minakata, Y. Morino, T. Ide, Y. Oderaotoshi and M. Komatsu, Chem. Commun., 2007, 3279 RSC.
- (a) Y. Kawashita, N. Nakamichi, H. Kawabata and M. Hayashi, Org. Lett., 2003, 5, 3713 CrossRef CAS PubMed; (b) H. Fujioka, K. Murai, Y. Ohba, A. Hiramatsu and Y. Kita, Tetrahedron Lett., 2005, 46, 2197 CrossRef CAS PubMed; (c) P. Gogoi and D. Konwar, Tetrahedron Lett., 2006, 47, 79 CrossRef CAS PubMed.
- S. Sayama, Synlett, 2006, 1479 CrossRef CAS PubMed.
- K. Schwekendiek and F. Glorius, Synthesis, 2006, 2996 CAS.
- S. Takahashi and H. Togo, Synthesis, 2009, 2329 CAS.
- P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- A. Corma and H. Garcia, Chem. Rev., 2002, 102, 3837 CrossRef CAS PubMed.
- R.-Y. Tang, Y.-X. Xie, Y.-L. Xie, J.-N. Xiang and J.-H. Li, Chem. Commun., 2011, 47, 12867 RSC.
- N. Panda and A. K. Jena, J. Org. Chem., 2012, 77, 9401 CrossRef CAS PubMed.
- (a) H. Jiang, H. Huang, H. Cao and C. Qi, Org. Lett., 2010, 12, 5561 CrossRef CAS PubMed; (b) C. Wan, L. Gao, Q. Wang, J. Zhang and Z. Wang, Org. Lett., 2010, 12, 3902 CrossRef CAS PubMed; (c) C. Wang, J. Zhang, S. Wang, J. Fan and Z. Wang, Org. Lett., 2010, 12, 2338 CrossRef PubMed.
- (a) J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS PubMed; (b) K. Karnakar, J. Shangkar, S. N. Murthy, K. Ramesh and Y. V. D. Nageshwar, Synlett, 2011, 1089 CAS.
- R. A. Kumar, C. U. Maheswari, S. Ghantasala, C. Jyothi and K. Rajender Reddy, Adv. Synth. Catal., 2011, 353, 401 CrossRef CAS PubMed.
- K. Rajender Reddy, C. Uma Maheswari, M. Venkateshwar and M. Lakshmi Kantam, Eur. J. Org. Chem., 2008, 3619 CrossRef PubMed.
- K. Rajender Reddy, C. Uma Maheswari, M. Venkateshwar and M. Lakshmi Kantam, Adv. Synth. Catal., 2009, 351, 93 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08578g |
| This journal is © The Royal Society of Chemistry 2014 |