Fast determination of phthalate ester residues in soft drinks and light alcoholic beverages by ultrasound/vortex assisted dispersive liquid–liquid microextraction followed by gas chromatography-ion trap mass spectrometry
Abstract
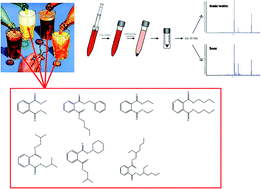
An ultrasound/vortex assisted dispersive liquid–liquid microextraction (USVADLLME) procedure coupled with gas chromatography–ion trap mass spectrometry (GC-IT-MS) is proposed for the rapid determination of seven phthalate esters in soft drinks and light alcoholic beverages (up to 6% alcohol by volume). Under the optimum conditions, the enrichment factors of the seven phthalate esters ranged from 205-fold to 315-fold for soft drinks and from 172-fold to 285-fold for light alcoholic beverages. The recoveries varied between 94.2% and 99.6% for soft drinks and 95.6% and 99.4% for light alcoholic beverages. The limits of detection were between 0.03 and 0.10 pg μL−1 and the limits of quantification were between 0.11 and 0.28 pg μL−1. The intra-day and inter-day precision expressed as the relative standard deviation varied between 2.9% and 5.1% and between 5.5% and 7.6%, respectively. The proposed USVADLLME-GC-IT-MS method was demonstrated to be simple, reproducible and practical for the determination of trace amounts of seven phthalate esters in soft drinks and light alcoholic beverages.

 Please wait while we load your content...
Please wait while we load your content...