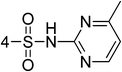
Synthesis and antibacterial evaluation of novel Schiff's base derivatives of nitroimidazole nuclei as potent E. coli FabH inhibitors†
Xin Zhang,
Chetan B. Sangani,
Li-Xin Jia,
Pi-Xian Gong,
Fang Wang,
Jun-Fang Wang and
Hai-Liang Zhu*
State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People's Republic of China. E-mail: zhuhl@nju.edu.cn; Fax: +86-25-83592672; Tel: +86-25-83592672
First published on 24th September 2014
Abstract
Series of novel Schiff's base derivatives have been synthesized by combining 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-formylbenzoate 5, 6 with aromatic/heterocyclic amine 7a–r, 8, 9a–r in ethanol. All compounds were evaluated for antibacterial assay and inhibition against E. coli FabH. Among the compounds studied, most of the compounds showed effective antibacterial and potential inhibitory activity against E. coli FabH. Compound 10q showed most potent inhibitory activity (IC50 = 2.6883 μM) by binding tightly to the active site of the E. coli FabH receptor with minimum binding energy (ΔGb = −55.3117 kcal mol−1), in which molecular docking study indicated the binding mode was stabilized by one hydrogen bond and five π–π interactions.
1 Introduction
An alarming increment in pathogenic resistance to existing first-line standard drugs is a serious problem in antimicrobial cure.1 Moreover, the progression of drug-resistant strains has contributed to the inefficiency of the straight antimicrobial therapy. This sets up an enormous interest in antibacterial research and we strongly believe that there is an urgent call for the development of novel antibacterial drugs with divergent and unique structures. Consequently, this area of research is accorded an enormous significance and continues to attract considerable attention from an increasing number of medicinal chemists. In order to prevent the serious medical problems caused by microorganisms, the discovery of new types of antibacterial agents is a crucial task at present. Fortunately, considerable research effort is made for the design of new antibacterial agents with high efficiency.2In the last 10 years, the research has been focused toward new antibacterial agents, which may act through different kinds of targets in key areas of the bacterial cell cycle, to surpass the problem of acquired resistance. The fatty acid synthesis (FAS) pathway in bacteria is a promising target in the recent research and fatty acid biosynthesis (FAB) is a fundamental metabolic process for microorganisms and essential for cell viability and growth.3,4
β-Ketoacyl-acyl carrier protein synthase III (FabH) is the key enzyme responsible for the first reaction in the pathway and plays an important regulatory role.5 FabH has also been demonstrated to be essential for initiating the fatty acid elongation cycles and is involved in the feedback regulation of the biosynthetic pathway via product inhibition.6,7 Some novel compounds had been demonstrated to inhibit FabH found in Gram-positive and Gram-negative bacteria, including multi-drug resistant strains. FabH proteins from Gram-positive and Gram-negative bacteria are highly conserved at the sequence and structural level whereas there are no significantly homologous proteins in humans. Importantly, the residues that comprise the active site are essentially invariant in various bacterial FabH molecules.8,9 FabH has been proved to be a promising target for the design of novel antimicrobial drugs because it adjusts and controls the fatty acid biosynthesis rate in an initiation pathway, and its substrate specificity is a key factor in membrane fatty acid composition.10–12 These facts indicate that small molecule inhibitors of FabH enzymatic activity could be potential candidates for selective, nontoxic, and broad spectrum antibacterials.
Because of varied biological activities, nitroimidazole derivatives have gained constant interests in drug research for antimicrobial chemotherapeutics and antiangiogenic hypoxic cell radiosensitizers. The metabolism and toxicology of nitroimidazole derivatives, particularly for secnidazole, have been characterized in recent reports.13,14 Secnidazole (α,2-dimethyl-5-nitro-1H-imidazole-1-ethanol) is extraordinarily effective in the treatment of giardiasis, amebiasis and bacterial vaginosis. By oral administration, secnidazole can be rapidly and completely absorbed, and has a longer terminal elimination half-life (17–29 h) than popular medication.15 Also, the treatment achieved with secnidazole is more effective and displays fewer side effects.16
Moreover, Schiff bases are compounds with the structure of AC = NB, which are usually synthesized from the condensation of active carbonyl groups and primary amines. Schiff's bases constitute an important class of biologically active drug molecules, which have attracted attention of medicinal chemists due to their wide range of pharmacological properties. These compounds are being synthesized as drugs by many researchers in order to combat diseases with minimal toxicity and maximal effects. These predictions have provided therapeutic pathways to develop new effective biologically active Schiff's base derivatives. Many researchers have studied the synthesis, characterization and structure-activity relationship (SAR) of Schiff bases and some Schiff bases were reported to have antibacterial activities.17 Moreover, Kim and co-workers reported the YKAs3003, a Schiff base condensed by 4-hydroxy salicylaldehyde and cyclohexanamine, as a potent inhibitor of Escherichia coli (E. coli) FabH with antimicrobial activity.18
Along the studies on FabH of our group based on nitroimidazole19 and Schiff's base,20 we report here the synthesis and structure-activity relationship of a new series of Schiff base derivatives of nitroimidazole nuclei in a single scaffold and their antibacterial activities against Escherichia coli, Pseudomonas aeruginosa (P. aeruginosa), Bacillus subtilis (B. subtilis) and Staphylococcus aureus (S. aureus) and also E. coli FabH inhibitory activities.
2 Results and discussion
2.1 Chemistry
Schiff's base derivatives 10a–r have been synthesized by reaction between the intermediate 5 (Intermediate 5 can be obtained by the simple two steps using compound 1 and metronidazole 3) and various substituted aniline 7a-r in ethanol and when refluxed overnight provides good yield (45–87%) (Scheme 1). Moreover, the addition of 2-phenylethanamine 8 into the intermediate 5 could produce target compound 11s in ethanol, when refluxed overnight. Other compounds 12t–w were derived from the reaction between compound 2 and secnidazole 4, subsequently interacting with four substituted aniline 9t–w in ethanol, refluxed overnight.The structures of all the new synthesized compounds were established by 1H NMR, elemental analysis, and molecular weight of compounds confirmed by mass spectrometry. Mass spectroscopy of compounds showed molecular ion peak (M+) corresponding to the exact mass.
2.2 Biological activity
| Compound | Minimum inhibitory concentrations (μg mL−1) of 10a–r, 11s, 12t–w | |||
|---|---|---|---|---|
| Gram-negative | Gram-positive | |||
| E. coli ATCC 35218 | P. aeruginosa ATCC 13525 | B. subtilis ATCC 6633 | S. aureus ATCC 6538 | |
| 10a | 25 | 100 | 25 | 50 |
| 10b | 6.25 | 12.5 | 3.13 | 6.25 |
| 10c | 25 | 100 | 25 | 50 |
| 10d | 12.5 | 100 | 25 | 25 |
| 10e | 50 | 100 | 25 | 100 |
| 10f | 3.13 | 12.5 | 3.13 | 6.25 |
| 10g | 6.25 | 25 | 3.13 | 12.5 |
| 10h | 6.25 | 100 | 12.5 | 12.5 |
| 10i | 25 | 100 | 25 | 50 |
| 10j | 12.5 | 100 | 12.5 | 25 |
| 10k | 6.25 | 12.5 | 3.13 | 6.25 |
| 10l | 3.13 | 25 | 12.5 | 25 |
| 10m | 12.5 | 50 | 6.25 | 25 |
| 10n | 12.5 | 50 | 12.5 | 25 |
| 10o | 3.13 | 12.5 | 6.25 | 6.25 |
| 10p | 6.25 | 25 | 3.13 | 6.25 |
| 10q | 1.56 | 3.13 | 1.56 | 3.13 |
| 10r | 3.13 | 12.5 | 6.25 | 12.5 |
| 11s | 6.25 | 25 | 6.25 | 12.5 |
| 12t | 12.5 | 25 | 3.13 | 12.5 |
| 12u | 12.5 | 100 | 12.5 | 25 |
| 12v | 3.13 | 25 | 3.13 | 12.5 |
| 12w | 3.13 | 12.5 | 6.25 | 12.5 |
| Penicillin G | 3.13 | 6.25 | 1.56 | 6.25 |
| Kanamycin B | 1.56 | 3.13 | 0.78 | 1.56 |
Upon investigation of antibacterial activity (Table 1), it has been observed that a majority of the compounds have shown effective activity against the strains used. Against Gram-negative bacteria E. coli, compound 10q (MIC = 1.56 μg mL−1) showed more effective activity compared to penicillin G (MIC = 3.13 μg mL−1) and comparable activity to kanamycin B (MIC = 1.56 μg mL−1), whereas compounds 10f, 10l, 10o, 10r, 12v and 12w (MIC = 3.13 μg mL−1) showed comparable activity to penicillin G (MIC = 3.13 μg mL−1). Against P. aeruginosa, compound 10q (MIC = 3.13 μg mL−1) showed comparable activity to kanamycin B (MIC = 3.13 μg mL−1). Against Gram-positive bacteria S. aureus, compound 10q (MIC = 3.13 μg mL−1) showed the most effective activity. As well compounds 10b, 10f, 10k, 10o and 10p (MIC = 6.25 μg mL−1) showed comparable activity as compared to penicillin G (MIC = 6.25 μg mL−1) but less compared to kanamycin B (MIC = 1.56 μg mL−1). Compound 10q (MIC = 1.56 μg mL−1) showed comparable activity and compounds 10b, 10f, 10g, 10k, 10p, 10t and 12v (MIC = 3.13 μg mL−1) showed less activity as compared to penicillin G (MIC = 1.56 μg mL−1) against B. subtilis. Of the compounds studied for E. coli FabH inhibitory activity (Table 2), compounds 10q (IC50 = 2.6883 μM), 12v (IC50 = 4.928 μM) and 10r (IC50 = 5.5923 μM) showed more potent activity as compared to secnidazole (IC50 = 28.5 μM) and metronidazole (IC50 = 17.6 μM) as well as other compounds of the series.
| Compounds | IC50 (μM) | Hemolysis LC30 (mg ml−1) |
|---|---|---|
| 10a | 31.4738 | >10 |
| 10b | 9.9087 | >10 |
| 10c | 39.0767 | >10 |
| 10d | 30.8408 | >10 |
| 10e | 31.0767 | >10 |
| 10f | 6.4994 | >10 |
| 10g | 12.2729 | >10 |
| 10h | 17.3407 | >10 |
| 10i | 43.4738 | >10 |
| 10j | 30.7389 | >10 |
| 10k | 11.1688 | >10 |
| 10l | 11.9535 | >10 |
| 10m | 14.8985 | >10 |
| 10n | 28.0284 | >10 |
| 10o | 6.1688 | >10 |
| 10p | 6.9193 | >10 |
| 10q | 2.6883 | >10 |
| 10r | 5.5923 | >10 |
| 11s | 10.0336 | >10 |
| 12t | 10.0857 | >10 |
| 12u | 28.4504 | >10 |
| 12v | 4.928 | >10 |
| 12w | 6.8125 | >10 |
| DCCP | 3.1542 | >10 |
Structure activity relationship (SAR) was carried out from E. coli FabH inhibitory and antibacterial activities. According to the activity data, it has been observed that the change in R substitution may lead to change in the activity against employed strains as well as E. coli FabH. Compounds 10o, 10p, 10q, 10r, 12v and 12w having sulfonamide linkage (–SO2NH) showed potent activity compared to other compounds. Electron releasing group R = 3-OCH3 in compound 10f gave better activity than 10e (R = 3-CH3), while there is no activity difference observed in 2-, 3- and 4-position of –CH3 group. Secnidazole derivatives 12t–w gave better activity than derivatives. Introduction of alkyl chain in compound 11s gave better activity than simple amine moiety in compound 10n; moreover, different halogen groups at 2-, 3- and 4-position gave different activity. The decreasing order of activity in halogen compounds is (4-Br > 4-F > 2-Cl > 2-Br > 4-Cl > 2, 4-Cl > 2-F). Moreover, reviewing and comparing the activity data, it is worthy to mention that the antibacterial activity against E. coli FabH of the target compounds depends not only on the heteroaromatic pharmacophore, but also on the nature of the substituents.
In addition, an acute oral toxicity test was conducted with mice to determine the toxicity from a single dose via the oral route. Based on the results (not listed), the single dose acute oral LD50 (half maximal concentration of lethal dose) values of the compounds (10b, 10f, 10q, 10r, 12v) are all greater than 5000 mg kg−1 of bodyweight.
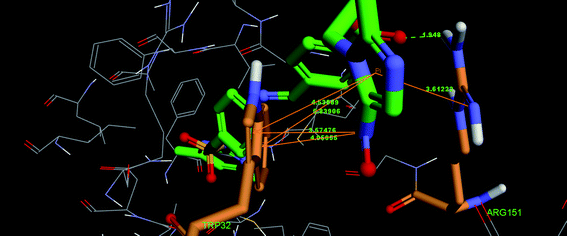
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl group) and ARG151 with distance: 1.948 Å; DHA angle: 133.0° and HAY angle: 149.3°, one π–π interaction formed between imidazole ring and ARG151 with distance 3.61222 Å, two π–π interactions formed between imidazolering and TRP32 with distance 4.53889 Å and 5.83906 Å and two π–π interactions formed between –NO2 group of imidazole ring and TRP32 with distance 3.57476 Å and 4.05855 Å. Moreover, the binding model of compound 10f and E. coli FabH is depicted in Fig. 3 and 4. In the binding model, compound 10f was nicely bound to the FabH kinase with two hydrogen bonds and one π–π interaction. A hydrogen bond formed between O-atom of –OCH3 and ARG151 with distance: 2.4297 Å; DHA angle: 107.1° and HAY angle: 100.2°, while another one formed between O-atom of C
O (carbonyl group) and ARG151 with distance: 1.948 Å; DHA angle: 133.0° and HAY angle: 149.3°, one π–π interaction formed between imidazole ring and ARG151 with distance 3.61222 Å, two π–π interactions formed between imidazolering and TRP32 with distance 4.53889 Å and 5.83906 Å and two π–π interactions formed between –NO2 group of imidazole ring and TRP32 with distance 3.57476 Å and 4.05855 Å. Moreover, the binding model of compound 10f and E. coli FabH is depicted in Fig. 3 and 4. In the binding model, compound 10f was nicely bound to the FabH kinase with two hydrogen bonds and one π–π interaction. A hydrogen bond formed between O-atom of –OCH3 and ARG151 with distance: 2.4297 Å; DHA angle: 107.1° and HAY angle: 100.2°, while another one formed between O-atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl group) and ASN247 with distance: 2.3913 Å; DHA angle: 125.3° and HAY angle: 117.0°. One π–π interaction formed between imidazole ring and HIS244 with distance 6.62666 Å. This molecular docking result, along with the biological assay data, suggests that compounds 10q and 10f prove to be potential inhibitors of E. coli FabH.
O (carbonyl group) and ASN247 with distance: 2.3913 Å; DHA angle: 125.3° and HAY angle: 117.0°. One π–π interaction formed between imidazole ring and HIS244 with distance 6.62666 Å. This molecular docking result, along with the biological assay data, suggests that compounds 10q and 10f prove to be potential inhibitors of E. coli FabH.
3 Conclusions
New Schiff's base derivatives 10a–r, 11s and 12t–w have been synthesized by reaction between 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-formylbenzoate 5, 6 and aromatic/heterocyclic amine in ethanol. This synthetic strategy allows the assimilation of two promising bioactive nuclei in a single scaffold through an easy method. Reviewing the biological activity data, it has been concluded that a majority of the compounds have been found most effective against applied bacterial strains. Compound 10q showed most effective inhibition by binding into the active site of E. coli FabH receptor with a minimum binding energy. According to this, it is worthy to mention that the Schiff's base derivatives having nitroimidazole nuclei have become a vital spot of antibacterial and E. coli FabH inhibition medicine research.4 Experiments
4.1 Materials and measurements
All chemicals and reagents used in the current work were of analytical grade. Melting points were determined using an XT4 MP apparatus (Taike Corp., Beijing, China). All the 1H NMR and 13C NMR spectra were recorded using a Bruker DPX300 model spectrometer in DMSO-d6 and chemical shifts were reported in ppm (d). ESI-MS spectra were recorded using a Mariner System 5304 mass spectrometer. Elemental analyses were performed using a CHN–O-Rapid instrument. TLC was performed using glass backed silica gel sheets (Silica Gel 60 GF254) and visualized in UV light (254 nm).4.2 General method for synthesis of Schiff's base derivatives
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-formylbenzoate 5, 6 (1 mol) and aromatic/heterocyclic amine 7a–r, 8, 9t–w (1.1 mol) were mixed together in ethanol as a solvent. The reaction mixture was stirred and refluxed overnight. After the completion of reaction (checked by TLC), the separated solid was filtered, washed well with ethanol (10 mL) and water (10 mL), and finally dried and recrystallized from ethanol to get the pure solid samples 10a–r, 11s, 12t–w. Physical, analytical, and spectroscopic characterization data of the compounds are presented hereafter.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).
N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).
N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).
N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).
N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).
N); anal. calcd for C21H20N4O4 (392.15 g mol−1): C, 64.28; H, 5.14; N, 14.28 (%); found: C, 64.49; H, 5.22; N, 14.33 (%); MS (m/z): 392.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).
N); anal. calcd for C21H20N4O5 (408.41 g mol−1): C, 61.76; H, 4.94; N, 13.72 (%); found: C, 61.80; H, 5.12; N, 13.82 (%); MS (m/z): 408.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17ClN4O4 (412.83 g mol−1): C, 58.19; H, 4.15; N, 13.57 (%); found: C, 58.25; H, 4.31; N, 13.62 (%); MS (m/z): 412.1 (M+).
N); anal. calcd for C20H17ClN4O4 (412.83 g mol−1): C, 58.19; H, 4.15; N, 13.57 (%); found: C, 58.25; H, 4.31; N, 13.62 (%); MS (m/z): 412.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17BrN4O4 (457.28 g mol−1): C, 52.53; H, 3.75; N, 12.25 (%); found: C, 52.65; H, 3.81; N, 12.42 (%); MS (m/z): 456.1 (M+).
N); anal. calcd for C20H17BrN4O4 (457.28 g mol−1): C, 52.53; H, 3.75; N, 12.25 (%); found: C, 52.65; H, 3.81; N, 12.42 (%); MS (m/z): 456.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17FN4O4 (396.37 g mol−1): C, 60.60; H, 4.32; N, 14.13 (%); found: C, 60.65; H, 4.40; N, 14.20 (%); MS (m/z): 396.4 (M+).
N); anal. calcd for C20H17FN4O4 (396.37 g mol−1): C, 60.60; H, 4.32; N, 14.13 (%); found: C, 60.65; H, 4.40; N, 14.20 (%); MS (m/z): 396.4 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17ClN4O4 (412.83 g mol−1): C, 58.19; H, 4.15; N, 13.57 (%); found: C, 58.25; H, 4.31; N, 13.62 (%); MS (m/z): 412.1 (M+).
N); anal. calcd for C20H17ClN4O4 (412.83 g mol−1): C, 58.19; H, 4.15; N, 13.57 (%); found: C, 58.25; H, 4.31; N, 13.62 (%); MS (m/z): 412.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17BrN4O4 (457.28 g mol−1): C, 52.53; H, 3.75; N, 12.25 (%); found: C, 52.65; H, 3.81; N, 12.42 (%); MS (m/z): 456.1 (M+).
N); anal. calcd for C20H17BrN4O4 (457.28 g mol−1): C, 52.53; H, 3.75; N, 12.25 (%); found: C, 52.65; H, 3.81; N, 12.42 (%); MS (m/z): 456.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H17FN4O4 (396.37 g mol−1): C, 60.60; H, 4.32; N, 14.13 (%); found: C, 60.65; H, 4.40; N, 14.20 (%); MS (m/z): 396.4 (M+).
N); anal. calcd for C20H17FN4O4 (396.37 g mol−1): C, 60.60; H, 4.32; N, 14.13 (%); found: C, 60.65; H, 4.40; N, 14.20 (%); MS (m/z): 396.4 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd For C20H16Cl2N4O4 (447.27 g mol−1): C, 53.71; H, 3.61; N, 12.53 (%); found: C, 53.82; H, 3.49; N, 12.43 (%); MS (m/z): 446.1 (M+).
N); anal. calcd For C20H16Cl2N4O4 (447.27 g mol−1): C, 53.71; H, 3.61; N, 12.53 (%); found: C, 53.82; H, 3.49; N, 12.43 (%); MS (m/z): 446.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C20H18N4O4 (378.38 g mol−1): C, 63.48; H, 4.79; N, 14.81 (%); found: C, 63.64; H, 4.63; N, 14.91 (%); MS (m/z): 378.1 (M+).
N); anal. calcd for C20H18N4O4 (378.38 g mol−1): C, 63.48; H, 4.79; N, 14.81 (%); found: C, 63.64; H, 4.63; N, 14.91 (%); MS (m/z): 378.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.70 (s, 1H, SO2NH); anal. calcd for C25H22N6O6S (534.54 g mol−1): C, 56.17; H, 4.15; N, 15.72 (%); found: C, 56.24; H, 3.93; N, 15.85 (%); MS (m/z): 534.1 (M+).
N), 11.70 (s, 1H, SO2NH); anal. calcd for C25H22N6O6S (534.54 g mol−1): C, 56.17; H, 4.15; N, 15.72 (%); found: C, 56.24; H, 3.93; N, 15.85 (%); MS (m/z): 534.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.72 (s, 1H, SO2NH); anal. calcd for C25H23N7O6S (549.56 g mol−1): C, 54.64; H, 4.22; N, 17.84 (%); found: C, 56.79; H, 4.12; N, 17.58 (%); MS (m/z): 549.1 (M+).
N), 11.72 (s, 1H, SO2NH); anal. calcd for C25H23N7O6S (549.56 g mol−1): C, 54.64; H, 4.22; N, 17.84 (%); found: C, 56.79; H, 4.12; N, 17.58 (%); MS (m/z): 549.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.75 (s, 1H, SO2NH); anal. calcd for C26H25N7O6S (563.59 g mol−1): C, 55.41; H, 4.47; N, 17.40 (%); found: C, 55.31; H, 4.25; N, 17.63 (%); MS (m/z): 563.2 (M+).
N), 11.75 (s, 1H, SO2NH); anal. calcd for C26H25N7O6S (563.59 g mol−1): C, 55.41; H, 4.47; N, 17.40 (%); found: C, 55.31; H, 4.25; N, 17.63 (%); MS (m/z): 563.2 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.73 (s, 1H, SO2NH); anal. calcd for C21H21N7O6S (499.50 g mol−1): C, 50.50; H, 4.24; N, 19.63 (%); found: C, 50.37; H, 4.06; N, 19.48 (%); MS (m/z): 499.1 (M+).
N), 11.73 (s, 1H, SO2NH); anal. calcd for C21H21N7O6S (499.50 g mol−1): C, 50.50; H, 4.24; N, 19.63 (%); found: C, 50.37; H, 4.06; N, 19.48 (%); MS (m/z): 499.1 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C22H22N4O4 (406.43 g mol−1): C, 65.01; H, 5.46; N, 13.78 (%); found: C, 64.86; H, 5.64; N, 13.85 (%); MS (m/z): 406.2 (M+).
N); anal. calcd for C22H22N4O4 (406.43 g mol−1): C, 65.01; H, 5.46; N, 13.78 (%); found: C, 64.86; H, 5.64; N, 13.85 (%); MS (m/z): 406.2 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C22H22N4O5 (422.43 g mol−1): C, 62.55; H, 5.25; N, 13.26 (%); found: C, 62.41; H, 5.04; N, 13.14 (%); MS (m/z): 422.2 (M+).
N); anal. calcd for C22H22N4O5 (422.43 g mol−1): C, 62.55; H, 5.25; N, 13.26 (%); found: C, 62.41; H, 5.04; N, 13.14 (%); MS (m/z): 422.2 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C22H22N4O4 (406.43 g mol−1): C, 65.01; H, 5.46; N, 13.78 (%); found: C, 65.13; H, 5.30; N, 13.83 (%); MS (m/z): 406.2 (M+).
N); anal. calcd for C22H22N4O4 (406.43 g mol−1): C, 65.01; H, 5.46; N, 13.78 (%); found: C, 65.13; H, 5.30; N, 13.83 (%); MS (m/z): 406.2 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.75 (s, 1H, SO2NH); anal. calcd for C26H25N7O6S (563.59 g mol−1): C, 55.41; H, 4.47; N, 17.40 (%); found: C, 55.31; H, 4.25; N, 17.63 (%); MS (m/z): 563.2 (M+).
N), 11.75 (s, 1H, SO2NH); anal. calcd for C26H25N7O6S (563.59 g mol−1): C, 55.41; H, 4.47; N, 17.40 (%); found: C, 55.31; H, 4.25; N, 17.63 (%); MS (m/z): 563.2 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.75 (s, 1H, SO2NH); anal. calcd for C22H23N7O6S (513.53 g mol−1): C, 51.46; H, 4.51; N, 19.09 (%); found: C, 51.52; H, 4.40; N, 19.20 (%); MS (m/z): 513.1 (M+).
N), 11.75 (s, 1H, SO2NH); anal. calcd for C22H23N7O6S (513.53 g mol−1): C, 51.46; H, 4.51; N, 19.09 (%); found: C, 51.52; H, 4.40; N, 19.20 (%); MS (m/z): 513.1 (M+).4.3 Biological assays
Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (HiMedia) at 37 ± 1 °C. The bacterial suspension was adjusted with sterile saline to a concentration of 1 × 104 to 105 CFU. The tested compounds and reference drugs were prepared by twofold serial dilution to obtain the required concentrations of 100, 50, 25, 12.5, 6.25 and 3.13 μg mL−1. The tubes were incubated in BOD incubators at 37 ± 1 °C for bacterial growth. The MICs were recorded by visual observations after 24 h (for bacteria) of incubation. Kanamycin B and penicillin G were used as standards for antibacterial activity. The observed MICs are presented in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm).
000 rpm).Harvested cells containing His-tagged ACP, ACPS, and FabHs were lysed by sonication in 20 mM Tris, pH 7.6, 5 mM imidazole, 0.5 M NaCl and centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The supernatant was applied to a Ni-NTA agarose column, washed, and eluted using a 5–500 mM imidazole gradient over 20 column volumes. Eluted protein was dialyzed against 20 mM Tris, pH 7.6, 1 mM DTT, and 100 mM NaCl. Purified FabHs were concentrated up to 2 mg mL−1 and stored at −80 °C in 20 mM Tris, pH 7.6, 100 mM NaCl, 1 mM DTT, and 20% glycerol for enzymatic assays.
000 rpm for 30 min. The supernatant was applied to a Ni-NTA agarose column, washed, and eluted using a 5–500 mM imidazole gradient over 20 column volumes. Eluted protein was dialyzed against 20 mM Tris, pH 7.6, 1 mM DTT, and 100 mM NaCl. Purified FabHs were concentrated up to 2 mg mL−1 and stored at −80 °C in 20 mM Tris, pH 7.6, 100 mM NaCl, 1 mM DTT, and 20% glycerol for enzymatic assays.
Purified ACP contains the apo-form that needs to be converted into the holo-form. The conversion reaction is catalyzed by acyl carrier protein synthase (ACPS). In the final volume of 50 mL, 50 mg ACP, 50 mM Tris, 2 mM DTT, 10 mM MgCl2, 600 μM CoA, and 0.2 μM ACPS were incubated for 1 h at 37 °C. The pH of the reaction was then adjusted to approximately 7.0 using 1 M potassium phosphate. Holo-ACP was purified by fractionation of the reaction mixture by Source Q-15 ion exchange chromatography using a 0–500 mM NaCl gradient over 25 column volumes.
In a final 20 μL reaction, 20 mM Na2HPO4/NaH2PO4, pH 7.0, 0.5 mM DTT, 0.25 mM MgCl2, and 2.5 μM holo-ACP were mixed with 1 nM FabH, and H2O was added to 15 mL. After 1 min incubation, a 2 μL mixture of 25 μM acetyl-CoA and 0.75 μCi [3H] acetyl-CoA was added for FabH reaction for 25 min. The reaction was stopped by adding 20 mL of ice-cold 50% TCA, incubating for 5 min on ice, and centrifuging to pellet the protein. The pellet was washed with 10% ice-cold TCA and re-suspended with 5 μL of 0.5 M NaOH. The incorporation of the 3H signal in the final product was read by liquid scintillation. When determining the inhibition constant (IC50), inhibitors were added from a concentrated DMSO stock such that the final concentration of DMSO did not exceed 2%.
4.4 Docking simulations
The crystal structures of E. coli FabH (PDB code: 1HNJ) were obtained from the Protein Data Bank (http://www.rcsb.org). Molecular docking of compounds into the three-dimensional X-ray structure of FabH was carried out using Ligand Fit Dock protocol of Discovery Studio 3.5.Acknowledgements
The work was financed by a grant (no. J1103512) from the National Natural Science Foundation of China.Notes and references
- N. Woodford, Expert Opin. Investig. Drugs, 2003, 12, 117–137 CrossRef CAS PubMed.
- Z. L. Li, Q. S. Li, H. J. Zhang, Y. Hu, D. D. Zhu and H. L. Zhu, Bioorg. Med. Chem., 2011, 19, 4413–4420 CrossRef CAS PubMed.
- J. Y. Lee, K. W. Jeong, J. U. Lee, D. I. Kang and Y. Kim, Bioorg. Med. Chem., 2009, 17, 5408–5413 CrossRef CAS PubMed.
- H. J. Zhang, Z. L. Li and H. L. Zhu, Curr. Med. Chem., 2012, 19, 1225–1237 CrossRef CAS.
- S. S. Khandekar, R. A. Daines and J. T. Lonsdale, Curr. Protein Pept. Sci., 2003, 4, 21–29 CrossRef CAS.
- J. T. Tsay, W. Oh, T. J. Larson and S. Jakowski, J. Biol. Chem., 1992, 267, 6807–6814 CAS.
- R. J. Heath and C. O. Rock, J. Biol. Chem., 1996, 271, 1833–1836 CrossRef CAS PubMed.
- C. E. Christensen, B. B. Kragelund, P. von Wettstein-Knowles and A. Henriksen, Protein Sci., 2007, 16, 261–272 CrossRef CAS PubMed.
- H. Q. Li, L. Shi, Q. S. Li, P. C. Lv, Y. Luo and H. L. Zhu, Bioorg. Med. Chem., 2009, 20, 6264–6269 CrossRef PubMed.
- R. Puupponen-Pimia, L. Nohynek, C. Meier, M. Kahkonen, M. Heinonen, A. Hopia and K. M. J. Oksman-Caldentey, Appl. Microbiol., 2001, 90, 494–507 CrossRef CAS.
- Y. Luo, L. R. Zhang, Y. Hu, S. Zhang, J. Fu, X. M. Wang and H. L. Zhu, ChemMedChem, 2012, 7, 1587–1593 CrossRef CAS PubMed.
- V. Brusic and N. Petrovsky, Exp. Rev. Clin. Immunol., 2005, 1, 145–157 CrossRef CAS PubMed.
- Y. Uto, H. Nagasawa, C. Z. Jin, S. Nakayama, A. Tanaka, S. Kiyoi, H. Nakashima, M. Shimamura, S. Inayama, T. Fujiwara, Y. Takeuchi, Y. Uehara, K. L. Kirk, E. Nakata and H. Hori, Bioorg. Med. Chem., 2008, 16, 6042–6053 CrossRef CAS PubMed.
- Y. Luo, H. Q. Li, Y. Zhou, Z. L. Li, T. Yan and H. L. Zhu, Chem MedChem, 2010, 5, 1117–1122 CrossRef PubMed.
- A. Boza, R. Gonzalez, H. Novoa, D. M. Cuéllar and M. Valdés, IL Farmaco, 2000, 55, 700–707 CrossRef.
- K. Soedin, O. Syukran, A. Fadillah and P. Sidabutar, Pharmaceutica, 1985, 4, 251–254 CAS.
- H. J. Zhang, X. Qin, K. Liu, D. D. Zhu, X. M. Wang and H. L. Zhu, Bioorg. Med. Chem., 2011, 19, 5708–5715 CrossRef CAS PubMed.
- J. Y. Lee, K. W. Jeong, J. U. Lee, D. I. Kang and Y. Kim, Bioorg. Med. Chem., 2009, 17, 1506–1513 CrossRef CAS PubMed.
- Y. T. Duan, Z. C. Wang, Y. L. Sang, X. X. Tao, S. B. Teraiya, P. F. Wang, Q. Wen, X. J. Zhou, L. Ding, Y. H. Yang and H. L. Zhu, Eur. J. Med. Chem., 2014, 76, 387–396 CrossRef CAS PubMed.
- F. Zhang, Q. Wen, S. F. Wang, B. S. Karim, Y. S. Yang, J. J. Liu, W. M. Zhang and H. L. Zhu, Bioorg. Med. Chem. Lett., 2014, 24, 90–95 CrossRef CAS PubMed.
- J. Mirzaei, M. Amini, H. Pirelahi and A. Shafiee, J. Heterocycl. Chem., 2008, 45, 921–925 CrossRef CAS.
- X. He, A. M. Reeve, U. R. Desai, G. E. Kellogg and K. A. Reynolds, Antimicrob. Agents Chemother., 2004, 48, 3093–3102 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08567a |
| This journal is © The Royal Society of Chemistry 2014 |