Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat
Abstract
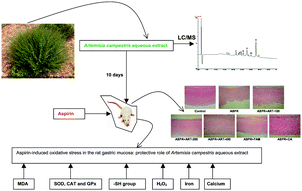
The present study aims at evaluating the antiulcer and antioxidant effect of Artemisia campestris aqueous extract (ACAE) as well as the mechanism of action involved in such gastroprotection. The use of LC/MS allowed the identification of 11 phenolic compounds and the colorimetric analysis demonstrated that the ACAE exhibited an important in vitro antioxidant activity. We first showed that in vivo ACAE protected against macroscopic and histological changes induced by aspirin in stomach mucosa. Aspirin administration was accompanied by an oxidative stress status assessed by an increase in malondialdehyde (MDA) level, a decrease in the content of sulfhydryl –(SH) groups and a depletion of antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). Pre-treatment with ACAE protected against aspirin-induced gastric oxidative stress. More importantly, aspirin administration increased plasma and tissue hydrogen peroxide (H2O2), free iron and calcium levels, while the ACAE pre-treatment reversed all the effects of aspirin-induced intracellular mediators. In conclusion, we suggest that Artemisia campestris aqueous extract has potent antiulcer and antioxidant properties. This gastroprotection offered by ACAE might be related partly to the safety of sulfhydryl group as well as its opposite effect on some intracellular mediators such as hydrogen peroxide, free iron and calcium.

 Please wait while we load your content...
Please wait while we load your content...